A case for importing limitation from specification into the claim
| April 6, 2019
Forest Laboratories, LLC v. Sigmapharm Laboratories, LLC
March 14, 2019
Before Prost, Dyk, and Moore (Opinion by Moore)
Summary
A district court’s narrowing claim interpretation that read a limitation from the specification into the claim may have helped a patent about an antipsychotic drug survive invalidity challenges. The Federal Circuit agreed with the district court’s claim interpretation, reasoning that repeated emphasis on the limitation in the specification and prosecution history supports the reading of the limitation into the claim. The Federal Circuit also agreed that, as a solution to an unrecognized problem, the claimed invention may not be obvious, particularly when there were no alternate, independent bases on which the prior art could be combined to make the claimed invention. Unfortunately, the district court’s lack of express findings on an unrelated proffered motivation to combine prompted the Federal Circuit to nevertheless vacate the district court’s nonobviousness determination and remand the case.
Details
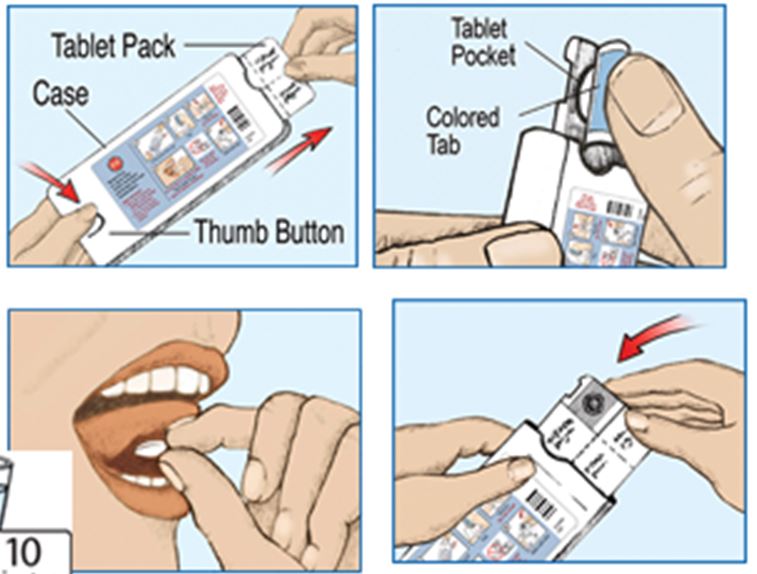
Forest Laboratories, LLC makes and sells the drug, SAPHRIS®, for treating bipolar disorders and schizophrenia. The active ingredient in SAPHRIS® is the compound, asenapine. This compound is the subject matter of U.S. Patent No. 5,763,476 (“476 patent”), also owned by Forest Laboratories.
SAPHRIS® on average costs almost $1,500 for 60 tablets, and there are currently no generic alternatives. When a group of drug makers sought approval from the FDA to make generic versions of SAPHRIS®, Forest Laboratories accused them of infringing the 476 patent.
For our purpose, claim 1 of the 476 patent is of particular interest:
1. A pharmaceutical composition comprising as a medicinally active compound: trans-5-chloro-2-methyl-2,3,3a,12b-tetrahydro-1H-dibenz[2,3:6,7]oxepino[4,5,-c]pyrrole or a pharmaceutically acceptable salt thereof; wherein the composition is a solid composition and disintegrates within 30 seconds in water at 37°C.
It is worth noting that claim 4 of the 476 patent recites a “method for treating tension, excitation, anxiety, and psychotic and schizophrenic disorders, comprising administering sublingually or buccally” the asenapine.
Among the issues on appeal, the Federal Circuit’s discussions on claim construction and nonobviousness of claim 1 are informative.
As to claim construction, the question was whether claim 1 should be limited to sublingually or buccally administered compositions, or as the accused infringers would argue, should cover any composition that meets the claimed disintegration profile.
The district court determined that claim 1 should be limited to sublingual or buccal compositions. And the Federal Circuit agreed.
Unlike claim 4, claim 1 does not expressly recite “sublingual or buccal” administrations. The original claim 1 did recite “[a] sublingual or buccal pharmaceutical composition…suitable for use in sublingual or buccal compositions”, but all the “sublingual or buccal” language was removed during prosecution.
At first glance, then, the district court’s claim interpretation would seem to contradict not only the plain language of the claims, and also the intrinsic evidence vis-à-vis the prosecution history.
However, both the district court and the Federal Circuit were able to draw for their claim interpretation from the specification and prosecution history of the 476 patent.
The 476 patent specification repeatedly uses “sublingual or buccal” to modify the “invention”. For example, the 476 patent is titled “sublingual or buccal pharmaceutical composition”, and statements like “the invention relates to a sublingual or buccal pharmaceutical composition” are peppered throughout the specification. The 476 patent also extolls sublingual and buccal treatments, and criticizes conventional peroral or oral treatments.
The Federal Circuit noted that “[w]hen a patent…describes the features of the ‘present invention’ as a whole, this description limits the scope of the invention” (quoting Verizon Servs. Corp. v. Vonage Holdings Corp., 503 F.3d 1295, 1308 (Fed. Cir. 2007). In addition, both the district court and the Federal Circuit cited UltimatePointer, LLC v. Nintendo Co., 816 F.3d 816 (Fed. Cir. 2016) to support their claim interpretation. In UltimatePointer, the court limited the claim term “handheld device” to a “direct-pointing device” (for example, a Wiimote), even though the claim language did not expressly contain such a limitation. “[T]he repeated description of the invention as a direct-pointing system, the repeated extolling of the virtues of direct pointing, and the repeated criticism of indirect pointing clearly point to the conclusion that the ‘handheld device’…is limited to a direct-pointing device.” Id. at 823-24.
The prosecution history of the 476 patent likewise repeatedly used “sublingual or buccal” to modify the claimed composition. Most notably, in interpreting the original claim 1, the Examiner took the position that the language “‘suitable for sublingually or buccal administration,’ does not result in a structural difference between the claimed invention and the prior art,” and the “the composition as claimed may be used for either mode of administration (sublingually or orally, rectally, etc.).” In response, the patentee amended claim 1 to define “suitability” to mean that “the composition is a solid composition and disintegrates within 30 seconds in water at 37°C”. This feature distinguished the claimed invention over the prior art:
The Office Action indicated that any composition whose physical characteristics make the composition unique to sublingual or buccal administration…would be allowable. Applicants submit that the distinguishing feature of disintegration time is exactly such a characteristic…It is this feature of rapid disintegration which distinguishes a sublingual composition from a peroral one and which makes the compositions of the present invention suitable to avoid the adverse effects observed with peroral administration….
To obtain the good effects of the compositions of the present invention, it is necessary that the medicine be delivered by sublingual or buccal administration.
The district court inferred from those statements the inventors’ intention to limit the claims to sublingual or buccal compositions. The Federal Circuit reached the same conclusion even without considering the prosecution history. Looking only at the specification, and in a rather attenuated logic, the Federal Circuit determined that since the specification describes the claimed disintegration time as defining “rapid disintegration”, and also describes “rapid disintegration” as a feature of sublingual/buccal composition, the claimed disintegration time must therefore limit the claim to “sublingual or buccal” compositions.
Based on the above interpretation, the district court and the Federal Circuit agreed that claim 1 of the 476 patent was not obvious.
Even though asenapine, the use of asenapine to treat schizophrenia, and sublingual and buccal administrations of drugs were separately known in the art, there was no motivation to combine the prior art to arrive at the 476 patent’s sublingual or buccal formulation.
The Federal Circuit noted a “problem/solution” basis for finding nonobviousness. “[W]here a problem was not known in the art, the solution to that problem may not be obvious”. “[S]olving an unrecognized problem in the art can itself be [a] nonobvious patentable invention, even where the solution is obvious once the problem is known.”
Here, the invention grew out of concerns over severe cardiotoxic side effects of oral asenapine, which caused cardiac arrest in some patients. This prompted the inventors to consider alternative routes of administration. However, the dangers of oral asenapine were unknown in the art at the time of invention. Large-scale clinical studies were even being conducted with conventional oral asenapine tablets.
In addition, the inventors found that the cardiotoxicity of oral asenapine was likely due to accumulation of unmetabolized asenapine. However, sublingual or buccal administrations were expected to produce more, not less, unmetabolized asenapine.
The district court found, and the Federal Circuit accepted, that since nothing in the prior art indicated that oral asenapine had problems, the person skilled in the art would not have been motivated to change the route of administration. Moreover, “it would not have been predictable or expected that sublingual administration would provide a solution to the problem of cardiotoxic effect.”
The accused infringers attempted to argue, as an alternate motivation to combine, the benefit of having more treatment options. However, the Federal Circuit dismissed this argument, because “a generic need for more antipsychotic treatment options did not provide a motivation to combine these particular prior art elements.”
The Federal Circuit did disagree with the district court on one thing—unexpected results. The district court found it unexpected that sublingual administrations of asenapine lacked the cardiotoxicity of the oral formulations, because the skilled person would have expected the contrary. However, if the problem was not known, then how could the solution to the problem be “unexpected”? There would have been no expectations. As the Federal Circuit explained, “[A] person of ordinary skill could not have been surprised that the sublingual route of administration did not result in cardiotoxic effects because the person of ordinary skill would not have been aware that other routes of administration do result in cardiotoxic effect”.
The fight, however, is not completely over. The accused infringers offered a different “motivation to combine” argument, based on whether sublingual or buccal administration would have addressed patient compliance problems. The Federal Circuit did not think the district court made sufficient express findings on this proffered motivation to combine, and for this reason, vacated the district court’s nonobviousness determinations and remanded the case.
The district court and the Federal Circuit’s “problem/solution” approach to nonobviousness in this case raised an interesting question. How does that approach reconcile with the established case law that any need or problem known in the field of endeavor at the time of invention and addressed by the patent can provide a reason for combining the prior art? The whole of MPEP 2144(IV) is dedicated to explaining how the motivation to combine can be for a purpose or problem different from that of the inventor.
The Federal Circuit answered that question in a recent, non-precedential decision in In re Conrad (Fed. Cir., March 22, 2019): “[C]ases found that the inventor’s discovery of and solution to an unknown problem weighed in favor of non-obviousness because the proffered reason to modify the prior art did not present a specific, alternate basis that was unrelated to the rationale behind the inventor’s reasons for making the invention.” Where the person skilled in the art would combine the prior art “for a reason independent from solving the problem identified by [the inventor]”, the fact that the invention solved an unrecognized problem may not lend as much patentable weight as it did in Forest Laboratories.
Takeaway
In this case, the narrower interpretation worked to the patentee’s advantage, particularly because the accused infringers had already conceded on the question of infringement. But such narrowing of the claim scope after the fact may not always be desirable. One may have preserved the validity of the patent, but is left with a negligible pool of potential infringers against whom to assert the patent.
Be mindful of how the invention is defined in the specification and during prosecution. Be careful with scope-limiting language such as “The present invention is…”, and absolute language such as “necessary”, “essential”, and “the distinguishing feature”.
Question the Examiner’s rationale for combining prior art. I have on several occasions seen Examiners use “more options” to rationalize a proposed combination of prior art. This may be a cop-out, because the Examiner may not have a specific, alternate basis for combining the prior art that is unrelated to the inventor’s reasons for making the invention. In that case, the nonobviousness of the invention may be formulated as the discovery of a solution to the “recognition of an unknown problem”.
Prior Art Based on Inherency Does Not Extend to “Probably” Existing Subject Matter, but Is Limited to “Necessarily” Existing Subject Matter.
| March 21, 2019
Personal Web Technologies, LLC v. Apple, Inc.
March 12, 2019
Before Moore, Taranto and Chen. Opinion by Chen.
Summary
In September, 2013, Apple, Inc. (Apple) filed an inter parties review (IPR) proceeding before the U.S. Patent Trial and Appeal Board (PTAB) against Personal Web Technologies, LLC (Personal Web) asserting unpatentability of U.S. Patent No. 7,802,310. The PTAB held that the ‘310 patent was obvious. On appeal, the Federal Circuit overturned the Board’s holding of obviousness on the basis that the Board had relied upon purported “inherent” subject matter in a prior art document without sufficient evidence that the purportedly inherent subject matter “necessarily exists” based on the teachings of the reference.
Details
Procedural Background
In September, 2013, Apple, Inc. (Apple) filed an inter parties review (IPR) proceeding before the U.S. Patent Trial and Appeal Board (PTAB) asserting multiple grounds of unpatentability of U.S. Patent No. 7,802,310 owned by Personal Web Technologies, LLC (Personal-Web). The PTAB held that the ‘310 patent was obvious over U.S. Patent No. 5,649,196 (Woodhill) in view of 7,359,881 (Stefik). Personal-Web appealed the PTAB’s ultimate determination of obviousness.
Factual Background
The ‘310 patent is directed to “Controlling Access to Data in a Data Processing System.” In the prior art, problems arose due to conventional naming techniques in which data files are typically identified by a name and/or pathname or location. In particular, with convention systems, when a data item is transferred from a first device to a second device, if the same data item exists in the second device a duplicate copy will be created on the second device.
According to the invention of the ‘310 patent, a data file or element is given a unique name by creating a “unique identifier” that is added to the data item’s identification along with its user-defined name, location, etc. In particular, this unique identifier is created by applying a cryptograph hash function to the data item, but creates a unique identifier for the specific data item. Accordingly, if the data item is different (e.g., modified), it will have a different unique identifier. However, if the data item is the same (e.g., not modified), it will have the same unique identifier.
The key patent claim at issue – independent claim 24 – is set forth below for reference. And, a key element of this claim at issue in this litigation is highlighted below.
24. A computer-implemented method implemented at least in part by hardware comprising one or more processors, the method comprising:
(a) using a processor, receiving at a first computer from a second computer, a request regarding a particular data item, said request including at least a content-dependent name for the particular data item, the content-dependent name being based, at least in part, on at least a function of the data in the particular data item, wherein the data used by the function to determine the content-dependent name comprises at least some of the contents of the particular data item, wherein the function that was used comprises a message digest function or a hash function, and wherein two identical data items will have the same content-dependent name; and
(b) in response to said request:
(i) causing the content-dependent name of the particular data item to be compared to a plurality of values;
(ii) hardware in combination with software determining whether or not access to the particular data item is unauthorized based on whether the content-dependent name of the particular data item corresponds to at least one of said plurality of values, and
(iii) based on said determining in step (ii), not allowing the particular data item to be provided to or accessed by the second computer if it is determined that access to the particular data item is not authorized.
In holding that the claim was obvious over U.S. Patent No. 5,649,196 (Woodhill) in view of 7,359,881 (Stefik), the PTAB held that the Woodhill reference inherently taught the highlighted features above.
Discussion
The Federal Circuit reversed the Board’s decision on the basis that the Board’s inherency finding determination of the above-noted highlighted feature lacked substantial evidence.
- The Federal Circuit’s Discussion of the Law
The Federal Circuit explained that “[w]hile it is possible that Woodhill’s system utilizes an un-stated Binary Object Identifier lookup table to locate binary objects of a previous version of a file that is going to be restored …, mere possibility is not enough.” The Court further indicated that “[i]nherency . . . may not be established by probabilities or possibilities” and that “[t]he mere fact that a certain thing may result from a given set of circumstances is not sufficient.”
The Federal Circuit explained that for inherency “a party must ‘show that the natural result flowing from the [disclosure] as taught would result in [the claimed subject matter]” – i.e., in this case that the “natural result flowing from the operation as taught would result in the performance of the questioned function.”
The Federal Circuit held that because the claimed subject matter “does not necessarily exist in the” Woodhill reference, reliance on inherency for that feature in the obviousness analysis was improper.
2. The Federal Circuit’s Discussion of the Evidence
In concluding that the Board’s inherency finding determination was not based on substantial evidence, the Federal Circuit explained that the Woodhill reference did not inherently include “causing the content-dependent name of the particular data item to be compared to a plurality of values” for the following reasons.
The Woodhill reference teaches a distributed management system in which files are apportioned into binary objects. The system uses binary object identifiers to determine whether a binary object has changed from one version of a file to the next, and only those binary objects whose content has changed needs to be backed up, thereby reducing the amount of data being backed up. In Woodhill, the binary object identifier is based on the contents of the binary object, such that the binary object identifier changes when the contents is changed.
At the PTAB, the Board had agreed with Apple’s argument that in order to determine which data needs to be restored in Woodhill by an update request, the system “must be able to” reference local data files using the binary object identification information received and, thus, that Woodhill must maintain some sort of file system or other mapping that allows the binary object identification record to serve as a lookup for the requisite file data that is to be restored. Thus, the Board asserted that Woodhill must inherently teach the disputed feature.
However, the Federal Circuit explained that “an equally plausible” understanding of Woodhill is that the system uses conventional file names and locations to locate files and the binary object information to locate a given binary object within the file. The Federal Circuit further explained that the only disclosed use of the binary object identifier in Woodhill is to perform a one-to-one comparison with the binary object identifier in the backed-up version of the object, which occurs after the object has been identified.
Takeaways
- This case emphasizes that inherency requires the “necessary” existence of subject matter that is not expressly shown or described in a reference. Accordingly, this case may be helpful in contesting rejections where asserted subject matter is not emphatically necessarily present, but deemed to be probable or likely by the Patent Office.
- This case also demonstrates that one way to
argue against a finding of inherency is to set forth an “equally plausible”
alternative that is supported by the reference.
That is, by demonstrating that an “equally plausible” alternative
subject matter may exist, it can be argued that the Patent Office cannot
conclude that the asserted subject matter inherently exist.
More than the mere look of a compound is required to establish obviousness
| January 22, 2019
Amerigen Pharmaceuticals v. UCB Pharma GMBH
January 11, 2019
Before Lourie, Chen and Stoll. Opinion by Lourie.
Summary
Amerigen appeals an inter parties review Decision by the U.S. Patent and trademark Office Patent Trial and Appeal Board (hereinafter “the Board”) that the U.S. Patent No. 6,858,650 (hereinafter ‘650) was not unpatentable as obviousness. The CAFC held that there was substantial evidence to support the Board’s findings. The CAFC affirmed the Board’s findings.
Details
The U.S. Patent No. 6,858,650 (hereinafter ‘650) claims some chemical derivative of 3,3‑diphenylpropylamines, including fesoterodine. Fesoterodine, an antimuscarinic drug with the following chemical structure:

“Fesoterodine is a prodrug of the active compound 5-hydroxymethyl tolterodine (hereinafter “5‑HMT”). A prodrug is an inactive molecule that, once inside the body, transforms to an active therapeutic agent.
5‑HMT is a metabolite of the compound tolterodine, an older antimusarinic drug….” Id. at 3. 5-HMT is an active metabolite, which has its own antimuscaranic activity and can contribute to the therapeutic effect of tolterodine. Tolterodine is converted to 5-HMT, once ingested by a patient, by cytochrome P450 2D6 (hereinafter “CYP2D6”). CYP2D6 converts the methyl group at the 5-position of the benzene ring of tolterodine to a hydroxylmethyl group in 5‑HMT. The following is the chemical structures of tolterodine and 5-HMT.
 A noted distinction between 5-HMT and fesoterodine is present at the 2-position of the benzene ring. In fesoterodine, an isobutyryl ester is at the 2-position. In 5-HMT, the 2-postion is a hydroxyl group.
A noted distinction between 5-HMT and fesoterodine is present at the 2-position of the benzene ring. In fesoterodine, an isobutyryl ester is at the 2-position. In 5-HMT, the 2-postion is a hydroxyl group.
An inter partes review (IPR) of ‘650 was brought by Mylan Pharmaceuticals Inc. The Board instituted the review based on “(1)obviousness over the Detrol Label, Postlind, Bundgaard, Bundgaard PCT, and Berge; and (2) obviousness over Brynne, Bundgaard, Bundgaard PCT, and Johansson.” Id. at 4. After the institution of the IPR, Amerigen joined as a party to the proceeding.
In the IPR, Petitioners argued that there was sufficient motivation for a skilled artisan to modify 5-HMT. Petitioners’ Expert testified that 5-HMT had insufficient lipophilicity and this would cause bioavailability problems. Id. at 7. The Expert, citing Brynne as support, stated that tolerodine is 10-fold more lipophilic than 5-HMT. UCB argued that there was no basis to allege modification of 5-HMT due to a bioavailability issue. UCB argued that none of the cited art suggested an issue regarding absorption by 5-HMT and further argued that “the lipophilicity of 5-HMT relative to tolterodine…did not show that 5-HMT had a bioavailability problem.” Id. at 7. Further, UCB’s Expert testified to an analysis of 5‑HMT using the Rule of 5 by Lipinski, which assesses 4 inherent properties of a compound to predict a potential bioavailability problem, and found that none of properties indicated a bioavailability problem for 5-HMT. Id. at 7. Petitioner’s Expert agreed that a skilled artisan would use the Rule of 5 to determine if there a potential bioavailability problem.
The Board held that, while a skilled artisan would have reason to choose 5-HMT as a lead compound, said artisan would not have been motivated to modify 5-HMT to achieve a prodrug wherein the 2-position hydroxyl group is replaced with an alkyl ester of six or fewer carbons. Id. at 6 and 7. Moreover, the Board, crediting UCB’s Expert, held that a skilled artisan would not have been motivated to modify 5‑HMT due to a bioavailability issue. Further, the Board, found that:
- Since there is no bioavailability issue, a skilled artisan would not have any reason or motivation to achieve a 5-HMT prodrug;
- Designing a prodrug is difficult since a skilled artisan must consider toxicity, bioavailability, and other drug characteristics of two compounds, rather than just one;
- Petitioners failed to establish that an ester of 5-HMT would be inactive, an essential characteristic of a prodrug; and
- Petitioners did not present any prodrugs in the same chemical class, with the same mechanism of action or the in the same field of treatment.
Id. at 8. The Board concluded that it would not have been obvious to develop a prodrug of 5‑HMT. Furthermore, the Board held that achieving the compounds claimed in the ‘650 patent would not have been a matter of routine optimization; none of the cited art disclosed the molecule fesoterodine and that there were too many possibilities of molecular modifications of 5-HMT consistent with a prodrug design to render obvious the compounds claimed in the ‘650 patent. The Board, relying upon UCB’s Expert, held that a skilled artisan may consider esterifying the hydroxy groups at the 2-position and the 5-postion, but that even if said artisan only considered esterification of the 2-position hydroxy, there was not reason or motivation to achieve an ester of six carbons or less and further, even if all possible esters were limited to only alkyl esters of 6 carbons or less at the 2-position, there would still be 86 possibilities disclosed. The Board held it would not be routine to test each of the 86 possibilities. Id. at 9.
Amerigen appealed the holding of the Board and argued before the CAFC that the Board’s holding was improper because:
- The Board did not understand the argument regarding lipophilicity and that a skilled artisan would have motivation to increase lipophilicity of 5-HMT for its own sake (motivation)
- An excessive burden was place upon Petitioner to establish a motivation to make 5-HMT a prodrug (burden)
- The Board did not give sufficient consideration to their routine optimization argument (routine optimization)
Id. at 16 and 17. Further, Amerigen argued that the Board did not give sufficient consideration to their argument regarding the effect of a patent claiming 5-HMT. UCB argued that Amerigen did not present a legal error committed by the Board and that the Board’s findings were supported by substantial evidence. (Side Note – UCB also argued that Amerigen lacked standing to bring an appeal. The CAFC held that Amerigen had standing. This issue is not addressed herein).
The CAFC affirmed the Board’s decision, finding that there was sufficient evidence to support their factual findings. “A finding is supported by substantial evidence if a reasonable mind might accept the evidence as adequate to support the finding. Consol. Edison Co. v. NLRB, 305 U.S. 197, 229 (1938).” Id. at 17.
Regarding Amerigen’s first argument (motivation), the CAFC concluded that there was substantial evidence to support the Board’s holding that there is no reason or motivation to modify 5-HMT to increase lipophilicity. The CAFC noted the Board’s holding that UCB’s Expert “better addressed the bioavailability issue and that lipophilicity of 5-HMT relative to tolterodine did not demonstrate a bioavailability problem. We agree with UCB that a reasonable fact finder could have weighted Dr. Roush’s (UCB Expert) testimony over Dr. Patterson’s (Petitioner’s Exert).” Id. at 19. The CAFC also noted the Rule of 5 assessment of 5-HMT by UCB’s Expert that found that there was “no reason to suspect that 5-HMT would possess poor oral absorption.” Id. at 19.
Before the CAFC, Amerigen argued that “there need not be a specific problem with bioavailability of 5-HMT for one of ordinary skill in the art to be motivated to modify 5-HMT to further improve its bioavailability.” Id. at 19. The CAFC determined that this was merely a conclusory argument that was insufficient to overcome the substantial evidence that supported the Board’s holding.
Regarding Amerigen’s second argument (burden), the CAFC held that the Board correctly found that Petitioners did not meet their burden of proof (“In an IPR, the petitioner has the burden of proving unpatentability by a preponderance of the evidence. 35 U.S.C. § 316(e).” Id. at 18.) Relying upon Bundgaard, the Board noted that a prodrug is an inactive drug. The Board also noted that Petitioners failed to present any evidence that an ester of 5-HMT would be inactive. The CAFC held that the “Board sensibly found that a skilled artisan would ‘seek some degree of certainty that a prodrug of a particular molecule would be inactive before embarking on the process of attempting to create the prodrug’ and the petitioners failed to provide any such certainty.” Id. at 21.
Before the CAFC, Amerigen argued that the Board impermissible placed too high of a burden on Petitioners by requiring them to present a prodrug that was analogous to 5-HMT. The Board had noted that Petitioners did not present any evidence of a prodrug that would be analogous to 5-HMT. The CAFC held that the Board did not commit an error. The Board did not require such evidence but merely found the lack of such evidence to further support UCB’s position. The CAFC held that the Board inquiring as to the existence or not of a prodrug, similar to the claimed compounds, is not a dispositive error.
Regarding Amerigen’s third argument (routine optimization), the CAFC held that there was sufficient substantial evidence to support the Board’s holding that the cited art did not render obvious achieving the claimed monoester substitution solely at the 2-position of a 5-HMT and that Amerigen did not establish a discernible error in the Board’s analysis.
The Board had found 1) in view of Bundgaard, a skilled artisan would consider diester substitutions as well as other prodrug moieties, 2) a skilled artisan would consider modifying the 5-position and/or the 2‑position, and 3) Bundgaard did not disclose isobutyryl ester of fesoterodine. The Board held it would not have been routine for a skilled artisan to achieve the claimed molecular modification to 5-HMT to achieve the claimed compounds of the ‘650 patent. Before the CAFC, Amerigen argued that “Bundgaard disclosed esters as prototypical prodrug moieties and that modifying the 2-position alone would have been the most obvious choice.” Id. at 22. The CAFC noted that the Board considered the disclosure of ester prodrugs by Bundgaard but also considered the disclosure by Bundgaard of many other prodrug substitutions. UCB’s Expert testified to at least 6 categories of additional substitutions. The Board held that modifications at the 5-position was as likely as modifications at the 2-position since the cited art “did not indicate a preference for either the 2- or 5-position, and the inventors themselves considered modifying the 5-position.” Id. at 22. The Board dismissed as unpersuasive testimony by Petitioner’s Expert that modifying only the 5-position would pose a risk of transesterification since the risk was not sufficiently explained.
Lastly, before the CAFC, Amerigen argued that the Board did not give sufficient weight to the Petitioner’s argument, presented in a single-sentence footnote in the petition, that a skilled artisan would have been motivated to modify 5-HMT because 5-HMT was patented at the time of invention. The CAFC held that even if a patent of 5-HMT provided a commercial motivation to modify 5‑HMT, such a motivation did not render the claimed compounds of the ‘650 patent obvious. The CAFC held that Amerigen did not meet its burden that “the ‘prior art would have suggested making the specific molecular modifications necessary to achieve the claimed invention.” Takeda Chem. Indus., Ltd. v. Alapharm Pty., Ltd., 492 F.3d 1350, 1356 (Fed. Cir. 2007) (emphasis added) (quoting In re Deuel, 51 F.3d 1552, 1558 (Fed. Cir. 1995)). A general motivation to modify 5-HMT based on a prior patent would not suffice….” Id. at 23.
The CAFC, affirming the holding of the Board, stated that “[A]ny compound may look obvious once someone has made it and found it to be useful, but working backwards from that compound, with the benefit of hindsight, once one is aware of it does not render it obvious.” Id. at 23.
Take away
- Ensure that there is a clearly identified reason for making the proposed modification. General assertions for making a proposed modification may not establish a prima facie case of obviousness
a. Ensure that the clearly identified reason is logical. Furthermore, ensure the alleged reason has a correlation to the cited feature.
2. Consider the breadth of the possibilities for a substitution presented in the cited art. The disclosure regarding a specific substitution may be exponentially greater when one considers more than one reference and/or more than one possible location of said substitution, e. 2‑position and/or 5-position of a benzene ring.
A claimed rule feature may not be anticipated and/or rendered obvious merely because the rule is satisfied by chance in a reference
| August 29, 2018
In re Facebook
August 14, 2018
Before Prost, Moore, Stoll. Opinion by Prost.
Summary
The CAFC reversed and remanded a PTAB decision, which had affirmed an Examiner’s anticipation and obviousness rejection of a patent application for a method of arranging images contiguously in an array. The CAFC held that anticipation of Applicants’ claimed rule is not established by an example in a cited reference, which just happens to satisfy Applicants’ claimed rule, since said rule is not satisfied all of the time in the cited reference.
Post-filing clarification of an ambiguous feature in a pre-filing reference is not sufficient to establish inherent properties of the feature in the earlier publication
| July 31, 2018
Endo Pharmaceuticals Solutions et al. v. Custopharm Inc.
July 16, 2018
Before Moore, Linn and Chen. Opinion by Chen.
Summary
Custopharm argued that Endo’s patents were invalid due to anticipation or obviousness of three features of a drug and its administration: the dosage, the content of the vehicle, and the administration schedule. The arguments regarding the dosage were rejected on the grounds that they relied on a lesser-used industry treatment guideline. The arguments regarding the vehicle were rejected on the grounds that they relied on an improper inherency position, and that it would not have been obvious to modify the vehicle in view of a reference teaching a similar vehicle in a different context. Finally, the arguments regarding the administration schedule were rejected on the grounds that they relied upon an unsupported claim construction position, as well as an improper combining of the teachings of two references.
Spray your way to non-obviousness: Patents directed to intranasal delivery of migraine drugs not obvious where prior art would have resulted in reduced efficacy
| July 11, 2018
Impax Laboratories, Inc. v. Lannett Holdings, Inc.
June 28, 2018
Before Lourie, Dyk, and Taranto. Opinion by Lourie.
Summary
This case involved a “close” question of obviousness that was ultimately decided in no small part by the perceived relative credibility of the parties’ experts. The Federal Circuit deferred to the district court’s preference for the patent owner’s expert, and consequently, the district court’s determination that, where the efficacy of a drug’s active ingredient depended not on itself, but on its more potent metabolite, a person of ordinary skill in the art would not have found it obvious to pursue a proposed combination of the prior art that would reduce the metabolite’s production.
The CAFC reviewed the PTAB’s application of the time-bar under § 315(b) and the obviousness determination
| June 20, 2018
WesternGeco LLC v. ION Geophysical Corporation, ION International S.A.R.L. (collectively, “ION”); In re WesternGeco, LLC.
May 7, 2018
Before Wallach, Chen and Hughes. Opinion by Chen.
This is the latest Federal Circuit (“CAFC”) decision in a series of patent litigations since 2009. In this case, the CAFC reviewed two main issues appealed from six inter partes review (“IPR”) decisions.
Firstly, the CAFC reviewed the Patent Trial and Appeal Board’s (“PTAB”) decision regarding the time-bar determination. Under § 315(b), the U.S. Patent and Trademark Office “may not institute an IPR where the petition ‘is filed more than 1 year after the date on which the petitioner, the real party in interest, or privy of the petitioner is served with a complaint alleging infringement of the patent.’” However, the PTAB found that ION was not sufficiently close to the third party, Petroleum Geo-Services, Inc. (“PGS”), “such that both should be bound by the trial outcome and related estoppels.” Thus, there was no privity between ION and PGS, and the time-bar was not applicable here. The CAFC affirmed the PTAB’s decision and this case was reviewed on the merits.
Secondly, the CAFC reviewed the PTAB’s determination of obviousness in the IPRs. Regarding U.S. Patent No. 7,080,607 (the “’607 Patent”), the CAFC affirmed the PTAB’s claim construction because WesternGeco’s argument relied on a part of the specification which only described preferred embodiment and could not rebut the PTAB’s rationale. With respect to U.S. Patent No. 7,293,520 (the “’520 Patent”), the CAFC said the PTAB’s obviousness determination was correct because WesternGeco failed to show evidence of impermissible hindsight to prove obviousness. As for U.S. Patent No. 7,162,967 (the “’967 Patent”), the CAFC affirmed the PTAB’s decision because the PTAB showed substantial evidence of obviousness which WesternGeco could not rebut. Also, WesternGeco did not show a nexus between the ’967 and ’520 Patents’ claims and any objective evidence of nonobviousness. The CAFC fully affirmed the PTAB’s conclusion.
Japanese Summary
本件は2009年に始まった特許訴訟で争われた一連の訴訟の最新のCAFC判決であり、CAFCは6つのIPRから控訴された2つの争点について判断した。
第一の争点は米国特許庁審判部(PTAB)による特許法第315条(b)項のTime-barの判断の正否であった。特許法第315条(b)項は、米国特許商標庁は、申立人、利害関係のある実際の当事者(real party in interest)、又は申立人と当事者関係(privity)のある者が当該特許の侵害を主張する告訴を受けた日から1年を超えて申立がなされた場合、IPRを開始することはできないと規定している。本件では、PTABは、ION社はPGS社のIPRの結果に拘束されるほど十分にPGS社と密接な関係をもっていないので、ION社とPGS社の間に当事者関係がないとした。CAFCはPTABの判断を支持し、本件の実体的事項を検討する(on the merits)とした。
第二の争点は、先のIPRにおけるPTABの自明性判断であった。607特許に関しては、WesternGeco社が自社の主張に引用した明細書の箇所は、実施例を述べているだけであり、PTABの解釈を反駁できなかったので、CAFCはPTABのクレーム解釈を認めた。520特許については、WesternGeco社は、PTABが「不当な後知恵(impermissible hindsight)」を用いたという証拠を示さなかったので、CAFCはPTABの自明性の判断を支持した。967特許については、PTABは自明である明白な証拠を示しており、WesternGeco社はそれに反論できなかった。さらに、WesternGeco社は967特許と520特許のクレームと、非自明性の証拠の因果関係(nexus)を示さなかったのでCAFCはPTABの解釈を支持した。
To Combine or not to Combine
| April 16, 2018
Ex parte Tesseir et al.
October 2, 2014
Before PTAB APJ Panel: Kerins, Staicovici & Woods.
Summary: The Board affirmed many of the rejections in this appeal of a Final Rejection. But the Board found that there was no motivation to support the obviousness rejection of claim 13 and therefore reversed the rejection of that claim.
要旨:
米国特許庁審判部は、最後の拒絶に対する本件アピール(拒絶査定不服審判に相当)に関し、審査官の拒絶理由の大部分を認めた。その一方、クレーム13に関する自明性の拒絶については、拒絶理由をサポートするだけの十分なモチベーション(動機付け又は理由付け)が存在しないと判断し、クレーム13に関する拒絶を覆した。具体的には、いわゆる後知恵を利用しなければ、審査官が主張するような引例の組み合わせを行なうだけの合理的な理由が存在しないと判断された。より具体的には、主引例は、既にリモート制御という構成要件を利用しており、特段の理由もなく、第2引例のリモート制御技術を組合わせるのは不合理であると判断された。
CAFC looks to complete disclosure of patent at issue and its related patents to counter patent owner’s arguments based on cherry-picking.
| February 8, 2018
Paice LLC v. Ford Motor Company
February 1, 2018
Before Lourie, O’Malley, and Taranto. Opinion by O’Malley
Summary
Due to error in the PTAB’s interpretation of incorporation by reference, the CAFC vacated the PTAB’s obviousness determination with respect to claims that relied on the incorporation by reference for their written description, and found substantial evidence to affirm the PTAB’s findings of obviousness of all other claims.
Tags: claim construction > Incorporation by reference > obviousness > written description
Bayer left hard up when CAFC reversed district courts final judgment with some stiff words for the lower court.
| November 9, 2017
Bayer Pharma AG, Bayer Intellectural Property GMBH, Bayer Heathcare Pharaceuticals, inc., v. Watson Laboratories Inc., Activis Pharma, Inc.
November 1, 2017
Before Lourie, Moore and O’Malley. Opinion by Moore
Summary:
The CAFC held that the district court clearly erred in finding that a skilled artisan would not have been motivated to arrive at claims 9 and 11 of the patent-in-suit.
The patent at issue is directed to a formulation of vardenafil and at least two sugar alcohols in the form of an uncoated oral disintegrating table (ODT). It was agreed by both parties that the claim covered an immediate-release formulation. Bayer markets a commercial embodiment of the patent under the name Staxyn, and its utility is erectile dysfunction.
« Previous Page — Next Page »

