“Kitchen” Sinks QuikTrip’s Trademark Opposition: The Federal Circuit Explains that Although Trademarks are Evaluated in their Entireties, when Determining Similarities between Trademarks, More Weight Is Applied to Distinctive Aspects of the Trademarks
WHDA Blogging Team | January 20, 2021
Quiktrip West, Inc. v. Weigel Stores, Inc
January 7, 2021
Lourie, O’Malley, and Reyna, (Opinion by Lourie)
Summary
QuikTrip filed a trademark opposition in the U.S. Patent & Trademarks Office (U.S.P.T.O.) against Weigel in relation to Weigel’s application for the trademark W Kitchens based on QuikTrip’s assertion that Weigel’s trademark is likely to cause confusion in relation to Quiktrip’s registered trademark QT Kitchen. The Board at the U.S.P.T.O. found that the dissimilarity of the marks weighed against the likelihood of confusion and dismissed QuikTrip’s opposition. On appeal, the Federal Circuit affirmed the Board’s decision. The Federal Circuit explained that the Board correctly evaluated the trademarks in their entireties, while properly giving lower weight to less distinctive aspects of the trademark, such as the term “kitchen.” The Federal Circuit also noted that although some evidence suggested that Weigel may have copied QuikTrip’s trademark, the fact that Weigel modified its trademark multiple times in response to QuikTrip’s allegations of infringement negated any inference of an intent to deceive or cause confusion.
Background
- The Factual Setting
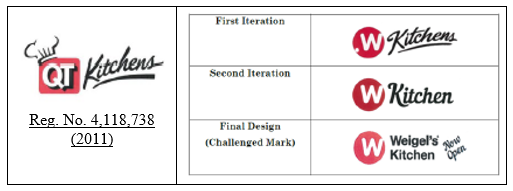
QuikTrip operates a combination gasoline and convenience store, and has sold food and beverages under its registered mark QT Kitchens (shown below left) since 2011.
In 2014, Weigel started using the stylized mark W Kitchens shown at the top-right below (First Iteration). QuikTrip sent Weigel a cease-and-desist letter. In response, Weigel adapted its mark to change Kitchens to Kitchen and to adapt the stylizing as shown at the middle-right below (Second Iteration). In response, QuikTrip demanded further modification by Weigel, and Weigel again adapted its mark to further change the font, to add its’ name Weigel’s and to include “new open” as shown at the bottom-right below (Final Challenged Mark).
The U.S.P.T.O. Proceedings
Weigel applied to register its’ Final Challenged Mark with the U.S. Patent & Trademark Office (application no. 87/324,199), and QuikTrip filed an opposition to Weigel’s mark under 15 U.S.C. 1052(d) asserting that it would create a likelihood of confusion with its QT Kitchens mark.
The Board evaluated the likelihood of confusion and found that the dissimilarity of the marks weighed against the likelihood of confusion and dismissed QuikTrip’s opposition to Weigel’s registration of its mark.
QuikTrip appealed the Board’s decision to the Federal Circuit.
The Federal Circuit’s Decision
The Federal Circuit reviews the Board’s legal determination de novo, but reviews the underlying findings of fact based on substantial evidence.
Here, the legal analysis involves the determination of whether the mark being sought is “likely, when used on or in connection with the goods of the applicant, to cause confusion” with another registered mark. See 15 U.S.C. 1052(d) of the Lanham Act. The likelihood of confusion evaluation is a legal determination based on underlying findings of fact relating to the longstanding factors set forth in E.I. DuPont de Nemours & Co., 476 F. 2d 1357 (C.C.P.A. 1973). For reference, these factors include:
- The similarity of the marks (e.g., as viewed in their entireties as to their appearance, sound, connotation, and commercial impression);
2. The similarity and nature of the goods and services;
3. The similarity of the established trade channels;
4. The conditions under which, and buyers to whom, sales are made (e.g., impulse
verses sophisticated purchasing);
5. The fame of the prior mark;
6. The number and nature of similar marks in use on similar goods (e.g., by 3rd
parties);
7. The nature and extent of any actual confusion;
8. The length of time during, and the conditions under which, there has been
concurrent use without evidence of actual confusion;
9. The variety of goods on which a mark is or is not used;
10. The market interface between the applicant and the owner of a prior mark;
11. The extent to which applicant has a right to exclude others from use of its mark;
12. The extent of possible confusion;
13. Any other established fact probative of the effect of use.
On Appeal, QuikTrip challenges the Board’s analysis regarding the Dupont factor number one (1) based on “similarity of the marks” and the Dupont factor thirteen (13) based on “other established fact probative of the effect of use” which other fact is asserted by QuikTrip as being the “bad faith” of Weigel.
- Factor 1 (Similarity of the Marks)
With respect to the first factor pertaining to the similarity of the marks, QuikTrip asserted that the Board incorrectly “dissected the marks when analyzing their similarity” rather than rendering its determination based on the similarity of “the marks in their entireties.”
First, QuikTrip argued that the Board improperly “ignored the substantial similarity created by … the shared word Kitchen(s)” and gave “undue weigh to other dissimilar portions of the marks.” In response, the Federal Circuit explained that “[i]t is not improper for the Board to determine that ‘for rational reasons’ it should give ‘more or less weight’ to a particular feature of the mark” as long as “its ultimate conclusion … rests on a consideration of the marks in their entireties.”
Here, the Federal Circuit indicated that the portion “Kitchen(s)” should “accord less weight” because “kitchen” is a “highly suggestive, if not descriptive, word” and also because there were numerous “third-party uses, and third-party registrations of marks incorporating the word “kitchen” for sale of food and food-related services.”
Moreover, the Federal Circuit explained that “the Board was entitled to afford more weight to the dominant, distinct portions of the marks” which included “Weigel’s encircled W next to the surname Weigel’s and QuikTrip’s QT in a square below a chef’s hat” emphasizing “given their prominent placement, unique design and color.”
The Federal Circuit also noted that the Board compared the marks in their entireties and further observe that: 1) the marks contained different letters and geometric shapes; 2) QuikTrip’s mark included a tilted chef’s hat; 3) phonetically, the marks do “not sound similar,” and the component Weigel’s adds an entirely different sound; and 4) the commercial impressions and connotations are different.
Accordingly, the Federal Circuit expressed that the Board’s finding of a lack of similarity was supported by substantial evidence.
- Factor 13 (Bad Faith as Another Probative Factor)
With respect to the thirteenth factor pertaining to asserted “bad faith” as being another probative factor, QuikTrip asserted that the evidence showed that Weigel acted in bad faith and that such bad faith further supported a finding of a likelihood of confusion.
However, the Federal Circuit explained that “an inference of ‘bad faith’ requires something more than mere knowledge of a prior similar mark.” Moreover, the Federal Circuit further explained that “[t[he only relevant intent is [an] intent to confuse.” The Federal Circuit noted that “[t]here is a considerable difference between an intent to copy and an intent to deceive.”
Accordingly, although the evidence showed that Weigel had taken photographs of QuikTrip’s stores and examined QuikTrip’s marketing materials, the Federal Circuit expressed that there was not an intent to deceive. In particular, the Federal Circuit emphasized that Weigel’s “willingness to alter its mark several times in order to prevent customer confusion [in response to QuikTrip’s cease-and-desist demands] negates any inference of bad faith.”
Accordingly, the Federal Circuit affirmed the decision of the Board.
Takeaways
- In evaluating similarities between trademarks for the determination of potential likelihood of confusion, although the evaluation is based on the marks in their entireties, different portions of the marks may be afforded different weight depending on circumstances. Accordingly, it is important to identify portions of the mark that are more distinctive in contrast to portions of the mark that may be descriptive or suggestive or commonly used in other marks.
- Although “bad faith” can help support a conclusion of a likelihood of confusion, it is important to keep in mind that “bad faith” does not merely require an “intent to copy,” but requires an “intent to deceive.”
- In the event that a trademark owner demands an alleged infringer cease-and-desist use of a mark, if the accused infringer substantially modifies its trademark in an effort to prevent customer confusion, according to the Federal Circuit, such action would “negate any inference of bad faith.” Accordingly, in order to help avoid any inference of bad faith, in response to a cease-and-desist demand, an accused infringer can help to avoid such an inference by modifying its trademark.
Tags: “DuPont Factors” Test > Likelihood of Confusion under 15 U.S.C. § 1052(d) > Similarity of Marks > Trademark
District court’s construction of a toothbrush claim gets flossed
WHDA Blogging Team | January 14, 2021
Maxill Inc. v. Loops, LLC (non-precedential)
December 31, 2020
Before Moore, Bryson, and Chen (Opinion by Chen).
Summary
Construing a claim reciting an assembly of identifiably separate components, the Federal Circuit determines that a limitation associated with a claimed component pertains to the component before it is combined with the other components. The lower court’s summary judgment of non-infringement, based on a claim construction requiring assembly, is reversed.
Details
Loops, LLC and Loops Flexbrush, LLC (“Loops”) own U.S. Patent No. 8,448,285. The 285 patent is directed to a flexible toothbrush that is safe for distribution in correctional and mental health facilities. The toothbrush being made of flexible elastomers is less likely to be fashioned into a shiv.
Claim 1 of the 285 patent is representative and is reproduced below:
A toothbrush, comprising:
an elongated body being flexible throughout the elongated body and comprising a first material and having a head portion and a handle portion;
a head comprising a second material, wherein the head is disposed in and molded to the head portion of the elongated body; and
a plurality of bristles extending from the head forming a bristle brush,
wherein the first material is less rigid than the second material.
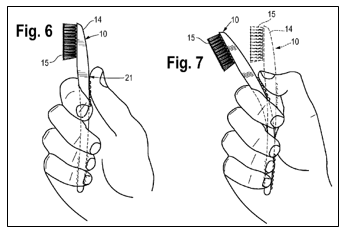
Maxill, Inc. makes “Supermaxx Prison/Institutional” toothbrushes that have a rubber-based, flexible body.

Loops and Maxill have for years been embroiled in patent infringement litigation, both in the U.S. and abroad, over flexible toothbrushes. This appeal arose out of a district court declaratory judgment action initiated by Maxill, who sought declaratory judgment of non-infringement and invalidity of the 285 patent.
During the district court proceeding, Loops moved for summary judgment of infringement. In their opposition, Maxill argued that whereas the “flexible throughout” limitation in the 285 patent claims required that the body of the toothbrush be flexible from one end to the other, only the handle portion of the body of the Supermaxx toothbrush was flexible. The head portion of the toothbrush body, including the head, did not bend.
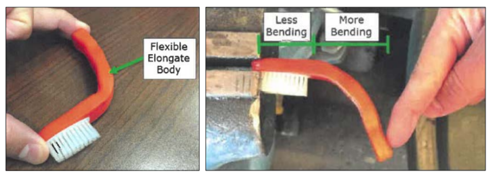
The district court agreed with Maxill, finding that Maxill’s Supermaxx toothbrush did not satisfy the “flexible throughout” limitation because the head portion of the toothbrush body, combined with the head, was rigid and unbendable. The district court then took the rare step of sua sponte granting Maxill summary judgment of non-infringement.
Loops appealed.
The main issue on appeal is the proper construction of the “flexible throughout” limitation. The question, however, is not simply whether the claim limitation requires that the body be flexible from one end to the other, but rather, whether the body must be flexible from one end to the other once assembled with the head.
As noted above, claim 1 of the 285 patent requires “an elongated body being flexible throughout the elongated body…and having a head portion and a handle portion”, and “a head…is disposed in and molded to the head portion of the elongated body”.
Does the “flexible throughout” limitation apply to the elongated body when it is combined with the head (i.e., post-assembly)? In that case, the district court was correct and Maxill’s Supermaxx toothbrush would not infringe the 285 patent.
Or does the “flexible throughout” limitation apply to the elongated body alone (i.e., pre-assembly)? In that case, the district court was wrong and Maxill’s Supermaxx toothbrush would be potentially infringing.
The Federal Circuit disagrees with the district court, determining that the “flexible throughout” limitation pertains to the elongated body alone and does not extend to the head even when the head is molded to the elongated body.
The Federal Circuit first looks at the claims, finding that the claims do not describe the head “as being a part of the elongated body; rather, the head…is identified in the claim as a separate element from the elongated body”.
Even though the claims require that the head be “disposed in and molded to” the elongated body, this recitation “does not meant that the head loses its identity as a separately identifiable component of the claimed toothbrush and somehow merges into becoming a part of the elongated body”,
The Federal Circuit then looks at the specification, finding that the specification consistently describes the head and elongated body as separate components.
Finally, the Federal Circuit finds that the prosecution history does not contain any “disclaimer requiring that the head be considered in evaluating the flexibility of the elongated body”.
The Federal Circuit is also bothered by the district court’s sua sponte summary judgment of non-infringement. This sua sponte action, says the Federal Circuit, deprived Loop of a full and fair opportunity to respond to Maxill’s non-infringement positions.
This case is interesting because the patentee seems to have accomplished a lot with (intentionally?) imprecise claim drafting. While the specification describes that the elongated body, as a standalone component, is fully flexible, there is no description that once combined with the head, the head portion of the elongated body remains flexible. But if the claims are construed the way the Federal Circuit has, written description or enablement is not an issue. And during prosecution, the patentee was able to distinguish over prior art teaching partially flexible toothbrush handles by arguing that the claimed elongated body was flexible throughout, but also distinguish over prior art disclosing toothbrushes with fully flexible handles and removable heads by arguing that the “permanent disposition of the head in the head portion is paramount in providing a fully flexible toothbrush while also providing the rigidity required in the head to effectively brush teeth”.
Takeaway
- Claim elements tend to be construed as identifiably separate components in the absence of claim language or descriptions in the specification indicating that the components are formed as a unitary whole. Take care when amending claims to add features that may exist only when the components are assembled or disassembled.
ALL PATENTABILITY ARGUMENTS SHOULD BE PRESENTED TO THE PTAB PRIOR TO AN APPEAL TO THE CAFC
Sung-Hoon Kim | January 6, 2021
In Re: Google Technology holdings LLC
November 13, 2020
Chen (author), Taranto, and Stoll
Summary:
The Federal Circuit affirmed the PTAB’s final decision that claims of Google’s patent application are unpatentable as obvious. The Federal Circuit found that since Google did not present its claim construction arguments before the PTAB in the first instance, Google forfeited these arguments and could not present again before the CAFC. The CAFC did not find any exceptional circumstances justifying considering these arguments before the CAFC.
Details:
Google Technology Holdings LLC (“Google”) appeals from the decision of the PTAB, where the PTAB affirmed the examiner’s final rejection of claims 1-9, 11, 14-17, 19, and 20 of the U.S. Patent Application No. 15/179,765 (‘765 application) under §103.
The ‘765 application is directed to “distributed caching for video-on-demand systems, and in particular to a method and apparatus for transferring content within such video-on-demand systems.” This application discloses a solution for how to determine streaming content to set-top boxes (STBs) and where to store the content among various content servers (video home offices (VHOs) or video server office (VSO)).
Claims 1 and 2 are representative claims of the ‘765 application and reproduced as follows:
1. A method comprising:
receiving, by a processing apparatus at a first content source, a request for content;
in response to receiving the request, determining that the content is not available from the first content source;
in response to determining that the content is not available from the first content source, determining that a second content source cost associated with retrieving the content from a second content source is less than a third content source cost associated with retrieving the content from a third content source, wherein the second content source cost is determined based on a network impact to fetch the content from the second content source to the first content source, . . .
2. The method of claim 1, further comprising:
determining that there is not sufficient memory to cache the content at the first content source; and
selecting one or more items to evict from a cache at the first content source to make available sufficient memory for the content, wherein the selection of the items to evict minimizes a network penalty associated with the eviction of the items, wherein the network penalty is based on sizes of the content and the items, and numbers of requests expected to be received for the content and the items.
Independent claim 1 recites a method of responding to requests to stream contents to STBs from content servers based on a cost of a network impact of fetching the content from the various content servers. Claim 2 recites a method of determining at which particular server(s) to store the content based on a network penalty.
During prosecution of the ‘765 application, the examiner rejected claim 1 in view of the Costa and Scholl references. The examiner rejected claim 2 in view of the Costa, Scholl, Allegrezza, and Ryu references.
PTAB
Google appealed the final rejection to the PTAB. Google argued that the cited references do not teach or suggest the claimed limitations (by “largely quoting the claim language and references” and “relying on block quotes from the claim language and the references”).
However, the PTAB affirmed the examiner’s obviousness rejections.
With regard to independent claim 1, the PTAB noted that because Google failed to cite a definition of “cost” or “network impact” in the specification that would preclude the examiner’s broad interpretation, the PTAB agrees with the examiner’s rejection that the combination of the cited references teaches or suggests the claimed limitation of independent claim 1.
With regard to claim 2, the PTAB noted that Google’s response were “conclusory,” and that Google “failed to rebut the collective teachings and suggestions of the applied references.” Instead, the PTAB argued that Google’s arguments focused merely on the shortcomings in the teachings of the cited references individually. Therefore, the PTAB agreed with the examiner.
Google did not introduce any construction of the term “network penalty” before the PTAB.
CAFC
First of all, the CAFC noted that “waiver is different from forfeiture.” United States v. Olano, 507 U.S. 725, 733 (1993). The CAFC noted that “whereas forfeiture is the failure to make the timely assertion of a right, waiver is the ‘intentional relinquishment or abandonment of a known right.’” Id. (quoting Johnson v. Zerbst, 304 U.S. 458, 464 (1938)).
Google argued to the CAFC that “[T]he Board err[ed] when it construed the claim terms ‘cost associated with retrieving the content’ and ‘network penalty’ in contradiction to their explicit definitions in the specification.” Therefore, Google argued that since the PTAB relied on the incorrect constructions of these two terms, the PTAB decision was not correct.
However, the CAFC noted that Google did not present these arguments to the PTAB. In other words, the CAFC noted that Google forfeited these arguments.
Google also argued to the CAFC that the CAFC should exercise its discretion to hear its forfeited arguments on appeal because (1) the PTAB sua sponte construed the term “cost”; and (2) the claim construction issue of “network penalty” is one of law fully briefed before the CAFC.
However, the CAFC noted that both arguments are not persuasive. The CAFC argued that Google did not provide any reasonable explanation as to why it never argued to the examiner during the prosecution or later to the PTAB. Also, the CAFC argued that since Google forfeited its argument for “network penalty” before the PTAB, Google’s second argument is not persuasive as well.
Therefore, the PTAB affirmed the PTAB’s decision upholding the rejection of the claims of the ‘765 application.
Takeaway:
- Applicant should make sure to present all arguments before the PTAB prior to an appeal to the CAFC.
- Factual issues v. legal issues: the CAFC applies significant deference to the PTAB on the factual issues (i.e., what the cited references teach and whether those teachings meet the claim limitations); the CAFC reviews legal questions without deference.
- There are benefits for an appeal to the CAFC: if the claim rejections are reversed, the prosecution is terminated, and the appealed claims are allowed without narrowing the claims (MPEP 1216.01 I.B and I.D).
New evidence submitted with a reply in IPR institution proceedings
WHDA Blogging Team | December 30, 2020
VidStream LLC v. Twitter, Inc.
November 25, 2020
Newman, O’Malley, and Taranto. Court opinion by Newman.
Summary
On appeals from the United States Patent and Trademark Office in IPR arising from two petitions filed by Twitter, the Federal Circuit affirmed the Board’s ruling that Bradford is prior art (printed publication) against the ’997 patent where the priority date of the ’997 patent is May 9, 2012 and a page of the copy of Bradford cited in Twitter’s petitions stated, in relevant parts, “Copyright © 2011 by Anselm Bradford and Paul Haine” and “Made in the USA Middletown, DE 13 December 2015.” The Federal Circuit affirmed the Board’s admission of new evidence regarding Bradford submitted by Twitter in reply (not included in petitions). The Federal Circuit also affirmed the Board’s rulings of unpatentability of claims 1– 35 of the ’997 patent over Bradford and other prior art references, in the two IPR decisions on appeal.
Details
I. background
U.S. Patent No. 9,083,997 (“the ’997 patent”), assigned to VidStream LLC, is directed to “Recording and Publishing Content on Social Media Websites.” The priority date of the ’997 patent is May 9, 2012.
Twitter filed two petitions for inter partes review (“IPR”), with method claims 1–19 of the ’997 patent in one petition, and medium and system claims 20–35 of the ’997 patent in the other petition. Twitter cited Bradford as the primary reference for both petitions, combined with other references.
With the petitions, Twitter filed copies of several pages of the Bradford book, and explained their relevance to the ’997 claims. Twitter also filed a Bradford copyright page that contains the following legend:
Copyright © 2011 by Anselm Bradford and Paul Haine
ISBN-13 (pbk): 978-1-4302-3861-4
ISBN-13 (electronic): 978-1-4302-3862-1
A page of the copy of Bradford cited in Twitter’s petitions also states:
Made in the USA
Middletown, DE
13 December 2015
VidStream, in its patent owner’s response, argued that Bradford is not an available reference because it was published December 13, 2015.
Twitter filed replies with additional documents, including (i) a copy of Bradford that was obtained from the Library of Congress, marked “Copyright © 2011” (this copy did not contain the “Made in the USA Middletown, DE 13 December 2015” legend); and (ii) a copy of Bradford’s Certificate of Registration that was obtained from the Copyright Office and contains following statements:
Effective date of registration: January 18, 2012
Date of 1st Publication: November 8, 2011
“This Certificate issued under the seal of the Copyright Office in accordance with title 17, United States Code, attests that registration has been made for the work identified below. The information on this certificate has been made a part of the Copyright Office records.”
Twitter also filed following declarations:
The Declaration of “an expert on library cataloging and classification,” Dr. Ingrid Hsieh-Yee, who declared that Bradford was available at the Library of Congress in 2011 with citing a Machine-Readable Cataloging (“MARC”) record that was created on August 25, 2011 by the book vendor, Baker & Taylor Incorporated Technical Services & Product Development, adopted by George Mason University, and modified by the Library of Congress on December 4, 2011.
the Declaration of attorney Raghan Bajaj, who stated that he compared the pages from the copy of Bradford submitted with the petitions, and the pages from the Library of Congress copy of Bradford, and that they are identical.
Twitter further filed copies of archived webpages from the Internet Archive, showing the Bradford book listed on a publicly accessible website (http://www.html5mastery.com/) bearing the website date November 28, 2011, and website pages dated December 6, 2011 showing the Bradford book available for purchase from Amazon in both an electronic Kindle Edition and in paperback.
VidStream filed a sur-reply challenging the timeliness and the probative value of the supplemental information submitted by Twitter.
The Patent Trial and Appeal Board (“PTAB” or “Board”) instituted the IPR petitions, found that Bradford was an available reference, and held claims 1–35 unpatentable in light of Bradford in combination with other cited references. Regarding Bradford, the Board discussed all the materials that were submitted, and found:
“Although no one piece of evidence definitively establishes Bradford’s public accessibility prior to May 9, 2012, we find that the evidence, viewed as a whole, sufficiently does so. In particular, we find the following evidence supports this finding: (1) Bradford’s front matter, including its copyright date and indicia that it was published by an established publisher (Exs. 1010, 1042, 2004); (2) the copyright registration for Bradford (Exs. 1015, 1041); (3) the archived Amazon webpage showing Bradford could be purchased on that website in December 2011 (Ex. 1016); and (4) Dr. Hsieh-Yee’s testimony showing creation and modification of MARC records for Bradford in 2011.”
VidStream timely appealed.
II. The Federal Circuit
The Federal Circuit unanimously affirmed all aspects of the Board’s holdings including Bradford being prior art (printed publication).
The critical issue on this appeal is whether Bradford was made available to the public before May 9, 2012, the priority date of the ’997 patent.
Admissibility of Evidence – PTAB Rules and Procedure
VidStream argued that Twitter was required to include with its petitions all the evidence on which it relies because the PTO’s Trial Guide for inter partes review requires that “[P]etitioner’s case-in-chief” must be made in the petition, and “Petitioner may not submit new evidence or argument in reply that it could have presented earlier.” Trial Practice Guide Update, United States Patent and Trademark Office 14–15 (Aug. 2018), https://www.uspto.gov/sites/de- fault/files/documents/2018_Revised_Trial_Practice_Guide. pdf.
Twitter responded that the information filed with its replies was appropriate in view of VidStream’s challenge to Bradford’s publication date, and that this practice is permitted by the PTAB rules and by precedent, which states: “[T]he petitioner in an inter partes review proceeding may introduce new evidence after the petition stage if the evidence is a legitimate reply to evidence introduced by the patent owner, or if it is used to document the knowledge that skilled artisans would bring to bear in reading the prior art identified as producing obviousness.” Anacor Pharm., Inc. v. Iancu, 889 F.3d 1372, 1380–81 (Fed. Cir. 2018).
The Federal Circuit sided with Twitter, concluding that the Board acted appropriately, for the Board permitted both sides to provide evidence concerning the reference date of the Bradford book, in pursuit of the correct answer.
The Bradford Publication Date
VidStream argued that, even if Twitter’s evidence submitted in reply were considered, the Board did not link the 2015 copy of Bradford with the evidence purporting to show publication in 2011, i.e., the date of copyright registration, the archival dates for the Amazon and other webpages, and the date the MARC records were created. VidStream argued that the Board did not “scrutiniz[e] whether those documents actually demonstrated that any version of Bradford was publicly accessible at that time.” VidStream states that Twitter did not meet its burden of showing that Bradford was accessible prior art.
Twitter responded that that the evidence established the identity of the pages of Bradford filed with the petitions and the pages from the copy of Bradford in the Library of Congress. Twitter explains that the copy “made” on December 13, 2015 was a reprint, for the 2015 copy has the same ISBN as the Library of Congress copy, as is consistent with a reprint, not a new edition.
After citing arguments of both parties, the Federal Circuit affirmed the Board’s ruling that Bradford is prior art against the ’997 patent because “[t]he evidence well supports the Board’s finding that Bradford was published and publicly accessible before the ’997 patent’s 2012 priority date.” There is no more explanation than this for the affirmance.
Obviousness Based on Bradford
The Federal Circuit affirmed the Board’s rulings of unpatentability of claims 1– 35 of the ’997 patent, in the two IPR decisions on appeal because VidStream did not challenge the Board’s decision of obviousness if Bradford is available as a reference.
Takeaway
· Although all relevant evidence should be submitted with an IPR petition, new evidence submitted with a reply may have chance to be admitted if the new evidence is a legitimate reply to the evidence introduced by a patent owner, or if it is used to document the knowledge that skilled artisans would bring to bear in reading the prior art identified as producing obviousness.
« Previous Page — Next Page »

