BREATHE EASIER, THE CAFC PROVIDES GUIDANCE ON CONSTRUING THE CLAIMED CONCENTRATION OF A STABILIZER IN PATENTS FOR AN ASTHMA DRUG
Andrew Melick | February 25, 2022
AstraZeneca v. Mylan Pharmaceuticals
Decided December 8, 2021
Taranto, Hughes, and Stoll. Opinion by Stoll. Dissent by Taranto
Summary:
AstraZeneca sued Mylan for infringement of its patents covering Symbicort pressurized metered-dose inhaler (pMDI) which is used for treating asthma. At issue in this case is how to construe the limitation “PVP K25 is present at a concentration of 0.001% w/w.” The district court construed the concentration using the ordinary standard scientific convention using only one significant figure to encompass the range from 0.0005% to 0.0014%. Based on this construction, Mylan stipulated to infringement. The district court then held a trial on validity and upheld the validity of the asserted claims. The CAFC affirmed the district court’s judgment of validity. But the CAFC disagreed with the construction of the concentration limitation. The CAFC held that the construction of the term should be narrower than the range provided by the ordinary meaning due to disclosures in the specification and arguments and amendments in the prosecution history. The CAFC construed the noted concentration of 0.001% to include the narrower range of 0.00095% to 0.00104%. Thus, the CAFC vacated the judgment of infringement and remanded for further proceedings on infringement.
Details:
The patents at issue in this case are U.S. Patent Nos. 7,759,328; 8,143,239; and 8,575,137 which cover the asthma inhaler Symbicort pMDI. A pMDI inhaler uses a propellant gas that is in liquid form under pressure in the pMDI device. When the inhaler is activated, the propellant causes the gas to come out as a spray making it easier to deliver the medication to the lower lungs. The representative claim is provided:
13. A pharmaceutical composition comprising
formoterol fumarate dihydrate, budesonide, HFA227, PVP K25, and PEG-1000,
wherein the formoterol fumarate dihydrate is present at a concentration of 0.09 mg/ml,
the budesonide is present at a concentration of 2 mg/ml,
the PVP K25 is present at a concentration of 0.001% w/w, and
the PEG-1000 is present at a concentration of 0.3% w/w.
Mylan appealed the district court’s stipulated judgment of infringement asserting that the district court erred in the construction of the concentration of PVP K25 of 0.001%. Mylan also appealed the district court judgement of nonobviousness.
The CAFC stated that as a matter of standard scientific convention, the noted concentration of 0.001% being expressed with only one significant digit would ordinarily encompass a range from 0.0005% to 0.0014%. AstraZeneca argued that this ordinary meaning controls absent lexicography or disclaimer. However, the CAFC stated that this is an acontextual construction that does not consider disclosures in the specification and arguments/amendments in the prosecution history. The CAFC stated that “[c]onsistent with Phillips, therefore, we must read the claims in view of both the written description and prosecution history.”
The CAFC stated that the testing evidence in the specification and prosecution history demonstrates that minor differences as small as ten thousandths of a percentage (four decimal places) in the concentration of PVP impact stability. The specification repeatedly describes formulations containing 0.001% PVP as providing the best suspension stability. This is also demonstrated in the data provided in the specification. The data includes formulations including PVP at concentrations of 0.0001%, 0.0005%, 0.001%, 0.01%, 0.03%, and 0.05%. The results show that the formulation containing 0.001% PVP was the most stable and that the formulation containing 0.0005% was one of the least stable formulations. The CAFC concluded that this data shows that PVP concentration of 0.001% is more stable than formulations with slight differences in PVP concentration (e.g., a concentration of 0.0005%, and that differences down to the ten-thousandth of a percentage (fourth decimal place) matters for stability.
In the prosecution history, the Applicant amended the claims to change ranges of the concentration to recite the exact concentration of PVP to 0.001% without using the qualifier “about,” and emphasized that 0.001% was critical to stability. The CAFC also stated that Applicant knew how to claim ranges and describe numbers with approximation using the term “about,” but the Applicant chose to claim the exact value. The CAFC concluded that this supports construing 0.001% narrowly, but that there should be some room for experimental error in the PVP concentration. The CAFC stated that the margin of error that is best supported by the intrinsic record is variations in the PVP concentration at the fourth decimal place (0.000095% to 0.000104%).
AstraZeneca argued that Mylan’s proposed construction is an attempt to limit the scope of the claims to the preferred embodiment. However, the CAFC stated that AstraZeneca’s proposed construction would read on two distinct formulations described in the specification (0.0005% PVP and 0.001% PVP), but the Applicant chose to claim only one of these formulations. The CAFC also stated that Applicant cancelled claims that included the 0.0005% PVP concentration and that this provides evidence that the formulations containing 0.0005% PVP are not within the scope of an asserted claim.
Regarding the nonobviousness determination by the district court, Mylan argued that the district court erred in finding that the prior art reference Rogueda taught away from the claimed invention. The CAFC citing Meiresonne v. Google, Inc., 849 F.3d 1379 (Fed Cir. 2017), stated that “a prior art reference is said to teach away from the claimed invention if a skilled artisan ‘upon reading the reference, would be discouraged from following the path set out in the reference, or would be led in a direction divergent from the path that was taken’ in the claim.”
The Rogueda reference included control formulations to compare with its novel formulations. Mylan relied on some of the control formulations as rendering the claims obvious. Rogueda disclosed that the novel formulations provided a “drastic” reduction in the amount of drug adhesion compared to the controls.
Mylan, citing Meiresonne, argued that the court’s precedent regarding “teaching away” is that a reference “that merely expresses a general preference for an alternative invention but does not criticize, discredit, or otherwise discourage investigation into the claimed invention does not teach away.” Mylan argued that Rogueda merely expresses a preference for the novel formulations over the control formulations citing a statement in the district court opinion that “Rogueda did not necessarily disparage the formulations in Controls 3 and 9.” However, AstraZeneca provided expert testimony stating that a skilled artisan looking at the adhesion results in Rogueda would conclude that the control formulations “were not suitable” and “clearly don’t work.” The district court credited the expert testimony that a skilled artisan would have known that the control formulations were unsuitable, and thus, discouraging investigation into these formulations, and concluded that Rogueda teaches away and does not render the claims obvious. The CAFC stated that there was no clear error in the district court’s determination and affirmed the validity of the claims.
Dissent
Judge Taranto dissented from the claim construction opinion. Judge Taranto stated that 0.001% should be construed to have its significant-figure meaning of 0.0005% to 0.0014% with the possibility of shrinking the lower end of the range to exclude the overlap area between the significant-figure interval of 0.001% (0.0005% to 0.0014%) and the significant-figure interval of 0.0005% (00045% to 0.00054%). Thus, if this exclusion is applied, the range would be 0.00055% to 0.0014%.
Judge Taranto takes the position that nothing in the specification or prosecution history warrants departing from the ordinary meaning. Judge Taranto pointed out that the specification, when referring to the formulations that were tested, states that several of the formulations were “considered excellent” and that the formulation with 0.001% PVP “gave the best suspension ability overall.” In the prosecution history, the Examiner stated the need for the Applicant to show criticality by showing unexpected results of a 0.001% concentration level compared to concentration levels “slightly greater or less” than 0.001% PVP. But it is unclear if the Examiner meant to include 0.0005% and other tested concentration levels tested within the meaning of “slightly greater or less.” The Examiner’s statement about criticality does not exclude 0.0005%.
Judge Taranto also stated that changing “0.001%” having one significant figure to “0.0010%” having two significant figures (as proposed by Mylan and adopted by the majority) requires rewriting the claim term, and that this rewriting is counter to the specification and prosecution history. The specification and prosecution history uniformly used only one significant figure when referring to PVP concentration. Just because four decimal places were used for some PVP concentrations does not mean that all concentration values should be read to express a degree of precision to four decimal places. The specification uses four decimal places to refer to the absolute concentration level in examples where this is unavoidable such as for 0.0001% and 0.0005%. However, absolute levels and degrees of precision are distinct.
Judge Taranto also addressed the fact that the range implied by 0.001% overlaps the range implied by 0.0005%. Judge Taranto stated that the overlap does not support adding an extra significant digit. Judge Taranto stated that “the most this overlap could possibly support would be an exclusion of the small range with the one significant-figure interval for which there is overlap resulting in the range of 0.00055% to 0.0014%. However, Judge Taranto pointed out that Mylan’s product is still within this narrower range, and thus, it is not necessary to decide whether the overlap-area exclusion is justified as a claim construction.
Comments
The majority appears to have been swayed by the fact that a formulation within the range of the ordinary meaning of the claimed concentration provided significantly different results. What is not clear is whether these different results, while not the best, could still be considered good. Judge Taranto pointed out that the specification stated that several of the tested formulations were considered excellent while the 0.001% was the best. When drafting and prosecuting patent applications, you need to be aware that your experimental data and amendments to the claims can be used to narrow the range afforded by the ordinary meaning of a certain value.
With regard to arguing non-obviousness due to a teaching away in the prior art, expert testimony can be critical and should be used to support your argument whether you are defending the validity of a patent or trying to invalidate.
REASONABLE-EXPECTATION-OF-SUCCESS REQUIREMENT CANNOT BE SATISFIED WHEN THERE IS NO EXPECTATION OF SUCCESS
Stephen G. Adrian | February 4, 2022
Teva Pharmaceuticals USA v. Corcept Therapeutics, Inc.
Decided December 7, 2021
Moore, Newman and Reyna (Opinion by Moore)
This precedential opinion illustrates the importance of preparing your expert for deposition. Although an expert declaration was effective in having a Post Grant Review instituted on claims 1-13 of U.S. Patent No. 10,195,214, the expert’s post-institution deposition testimony was instrumental in upholding the validity of the claims. This opinion not only addresses obviousness with respect to the reasonable expectation of success requirement, but also obviousness of ranges, a topic covered by Michael Caridi earlier this month in Indivior UK Ltd., v. Dr. Reddy’s Labs S.A., Dr. Reddy’s Labs, Inc.
Mifepristone was developed as an anti-progestin in the 1980s and was later discovered to likely inhibit the effect of cortisol on tissues, suggesting that it could be used to treat Cushing’s syndrome (a disease caused by excessive levels of cortisol). Corcept filed for a New Drug Application (NDA) for Korlym (a 300 mg mifepristone tablet) to control hyperglycemia secondary to hypercortisolism in certain patients with Cushing’s syndrome. The NDA application was approved with a few post marketing requirements, one including a drug-drug interaction clinical trial to determine a quantitative estimate of the change in exposure of mifepristone following co-administration of ketoconazole (a strong CYP3A inhibitor). A memorandum provided by the FDA explained that “[t]he degree of change in exposure of mifepristone when co-administered with strong CYP3A inhibitors is unknown. . .”.
In approving Corcept’s NDA, the FDA also approved the Korlym label recommending a dose of 300 mg once daily and allowing for increasing dosage in 300 mg increments to a maximum 1200 mg once daily. In addition, the label warned against using mifepristone with strong CYP3A inhibitors and limited the dose to 300 mg per day when used with strong CYP3A inhibitors.
Corcept conducted the drug-drug interaction study and collected data on co-administration of mifepristone with a strong CYP3A inhibitor and received the ‘214 patent.
Teva sought post-grant review after Corcept asserted the ‘214 patent in district court, arguing that the claims would have been obvious based on the Korlym label and the FDA memorandum, optionally in combination with FDA guidance on drug-drug interaction. Furthermore, Teva provided an expert declaration of Dr. Greenblatt opining that it was reasonably likely that 600 mg mifepristone would be well tolerated and therapeutically effective when co-administered with a strong CYP3A inhibitor.
The Patent Trial and Appeal Board (PTAB) found that Teva had failed to prove that claims 1-13 would have been obvious. The PTAB construed the claims to require safe administration of mifepristone, and that Teva failed to show that one of ordinary skill in the art would have had a reasonable expectation of success for safe co-administration of more than 300 mg mifepristone with a strong CYP3A inhibitor.
Teva asserted that the PTAB had committed two errors: (1) that the PTAB erred in requiring precise predictability rather than a reasonable expectation of success, and (2) that the PTAB erred when it found Teva had failed to prove the general working conditions (ranges) disclosed in the prior art encompassed the claimed invention. The CAFC did not agree.
1. Reasonable Expectation of Success
The reasonable expectation of success analysis must be tied to the scope of the claims.
Claim 1 is representative:
A method of treating Cushing’s syndrome in a patient who is taking an original once-daily dose of 1200 mg or 900 mg per day of mifepristone, comprising the steps of:
reducing the original once-daily dose to an adjusted once-daily dose of 600 mg mifepristone,
administering the adjusted once-daily dose of 600 mg mifepristone and a strongCYP3A inhibitor to the patient,
wherein said strong CYP3A inhibitor is selected from the group consisting of ketoconazole, itraconazole, nefazodone, ritonavir, nelfmavir, indinavir, boceprevir, clarithromycin, conivaptan, lopinavir, posaconazole, saquinavir, telaprevir, cobicistat, troleandomycin, tipranivir, paritaprevir, and voriconazole.
The claims require safe co-administration of a specific amount of mifepristone with a strong CYP3A inhibitor. Teva failed to establish that one would have reasonably expected co-administration of more than 300 mg mifepristone with a strong CPY3A inhibitor to be safe for treatment of Cushing’s syndrome. The PTAB went further to find that one of ordinary skill in the art would have had no expectation of success. Although not in the CAFC’s opinion, the deposition testimony of Teva’s expert (Dr. Greenblatt) was devastating.From the deposition:
[Patent Owner’s Counsel]: Well, okay. With all those assumptions built into this, was this — was the result set forth here that there was little to no increase in adverse events, was that result predictable?
[Dr. Greenblatt]: It’s — the result is neither predictable or unpredictable. It is what it is. It’s the study was done as mandated by the FDA to get at the truth, and here’s the outcome of the study.
[Patent Owner’s Counsel]: So a person of skill in the art would not have expected there to be no increase in adverse events?
[Dr. Greenblatt]: The study was done to, in part, to answer that question, not to address an expectation. I don’t believe that there would be any expectation. You don’t know what’s going to happen, which is why you do the study.
[Patent Owner’s Counsel]: So if the same testing had shown that a dose of 600 mg mifepristone could not be safely administered with ketoconazole, would that have been expected by a person of skill in the art?
. . .
[Dr. Greenblatt]: Yeah, the same answer. I don’t think there’s an expectation. You’re doing the study to find out what the result is to get the scientific truth.
The PTAB had discredited Dr. Greenblatt’s pre-institution testimony based on this post-institution testimony which unequivocally stated that one of ordinary skill would have no expectation as to whether 600 mg of mifepristone and ketoconazole would be safe. 2. Prior Art Range Precedence
Teva had asserted that the sole administration of mifepristone up to 1200 mg was an overlap in the range required by claim 1. This argument failed because the evidence of recorded only supported that the general working conditions for co-administration was shown to be 300 mg/day. As such, there was no overlap. Thus. The PTAB’s finding that the prior art ranges do not overlap was supported by substantial evidence.
Takeaways
- Make sure your expert is prepared.
- Obviousness based on the reasonable-expectation-of-success analysis must be tied to the scope of the claims.
- General working conditions in the prior art need to be analyzed with respect to the scope of the claims.
Federal Circuit Restricts Ranges for lack of Written Description
Michael Caridi | January 19, 2022
Indivior UK LTD., v. Dr. Reddy’s Labs S.A., Dr. Reddy’s Labs, Inc.
Decided on November 24, 2021
Lourie, Linn and Dyk. Opinion by Lourie. Dissent by Linn.
Summary
The Federal Circuit Court affirmed a Patent Office ruling for an IPR finding that there is lack of written description support in an application for ranges in claims of a continuation patent thereby resulting in the claims being anticipated by prior art. The Court found that the Tables in the application, relied on for support, lacked sufficient clarity as the values therein did not constitute ranges but only specific, particular examples.
Details
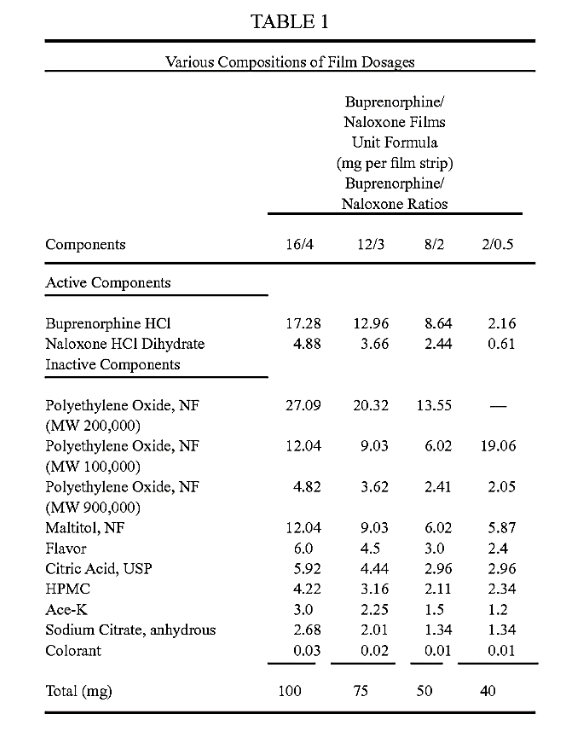
Indivior owns USP 9,687,454 (the ’454 patent), directed to orally dissolvable films containing therapeutic agents. The ’454 patent issued as the fifth continuation of an application filed on August 7, 2009 to which Indivior claimed the filing date.
Dr. Reddy’s Labs (DRL) petitioned for inter partes review of claims 1–5 and 7–14 at the PTAB alleging that the polymer weight percentage limitations, added to the claims by amendment, do not have written description support in the application as filed and thus are not entitled to the benefit of its filing date. Specifically, the limitation in question of claim 1 reads:
…. about 40 wt % to about 60 wt % of a water-soluble polymeric matrix;…
Also in question was claim 8’s polymer weight percentage limitation of “about 48.2 wt %,” which the Board found that Tables 1 and 5 in the application disclose formulations from which a polymer weight of 48.2% could be calculated by a person of ordinary skill in the art.
Regarding claims 1, 7 and 12 recitations of polymer weight percentage limitations as ranges: “about 40 wt % to about 60 wt %” (claim 1) and “about 48.2 wt % to about 58.6 wt %” (claims 7 and 12), the Board found that the application does not “discuss or refer to bounded or closed ranges of polymer weight percentages.” The Board also found that a person of ordinary skill would have been led away from a particular bounded range by the application’s teaching that “[t]he film may contain any desired level of self-supporting film forming polymer.” Based thereon, the Board determined that claims 1–5, 7, and 9–14 do not have written description support in the application.
Indivior appealed the Board’s decision as to claims 1, 7 and 12 while DRL cross-appealed as to the Board’s decision as to claim 8.
Discussion
Indivior’s Appeal – Claims 1, 7 and 12
Indivior argued that the Board erred in finding that the polymer range limitations in claims 1, 7, and 12 lack written description support in the application. Indivior specifically argued that Tables 1 and 5 disclose formulations with 48.2 wt % and 58.6 wt % polymer and that the application also discloses that “the film composition contains a film forming polymer in an amount of at least 25% by weight of the composition.” Indivior argued that the combination of these disclosures encompasses the claimed ranges. DRL, countered that the application does not disclose any bounded range, only a lower endpoint and some exemplary formulations from which a skilled artisan would not have discerned any upper range endpoint.
Regarding claim 1, the Court agreed with the Board that there is no written description support in the application for the range of “about 40 wt % to about 60 wt %.” The Court noted that the range was not expressly claimed in the application; nor are the values of “40 wt %” and
“60 wt %” and a range of 40 wt % to 60 wt %.
The CAFC went on to further note that not only was there no express description of these values, various other indications of the polymeric content of the film are present in the application, rendering it even less clear that an invention of “about 40 wt % to about 60 wt %” was contemplated as an aspect of the invention. Most specifically, the Court reiterated the PTAB’s reliance on the application’s paragraph 65 statement that “[t]he film may contain any desired level of . . . polymer.” The Court asserted that this statement is contrary to Indivior’s assertion that the level of polymer should be closed and between “about 40 wt % to about 60 wt %.” of claim 1.
Regarding the disclosures in Tables 1 and 5 of the application, the Court noted that in Table 1 there are four polymer components of the described formulations, polyethylene oxide, NF (MW 200,000); polyethylene oxide, NF (MW 100,000); polyethylene oxide, NF (MW 900,000); and HPMC, and when they are added up, each total is within the “about 40 wt % to about 60 wt %” range. However, the CAFC found that these values do not constitute ranges but only specific, particular examples.
Thus, the Court concluded that this was insufficient written description support for the claimed ranges, stating:
Here, one must select several components, add up the individual values, determine the aggregate percentages, and then couple those aggregate percentages with other examples in the ’571 application to create an otherwise unstated range.
As such the CAFC affirmed that the Board properly determined that claims 1, 7, and 12 do not have written description support in the application and are therefore anticipated by the prior art asserted during the IPR.
DLR’s Cross-Appeal – Claim 8
Regarding claim 8, which the Board had found sufficient written description for, the Court affirmed that determination, even though, as DRL argued, the number “48.2 wt %” is not explicitly set forth in the application. The Court noted that “even though one might see some inconsistency” between this result and their ruling as to claims 1, 7 and 12 since claim 8 does not recite a range, but only a specific amount, this can be derived by selection and addition of the amounts detailed in Tables 1 and 5 of the application.
Dissent
Judge Linn dissented from the majority as to affirming the Board’s decision on Indivior’s appeal, specifically taking exception to the majority’s interpretations of the often-cited written description cases In re Wertheim and Nalpropion.
Linn particularly noted that the majority too narrowly interpreted the disclosures of the application. Specifically, Linn referred to the noted paragraph 65 of the application as a truncated text “[t]he film may contain any desired level of … polymer” to wrongly suggest that the statements about film polymer levels of “at least 25%” or “at least 50%” fail to provide clear support for the claimed “about 40 wt % to about 60 wt %” range. He asserts that the quoted passage is taken out of context and ignores the remaining part of the sentence, which expressly links the aggregate polymer percentage to the key claimed characteristics of mucoadhesion and rate of film dissolution shared by films having the stated polymer levels. He quotes the full text from paragraph 65 noting that it states that “any film forming polymers that impart the desired mucoadhesion and rate of film dissolution may be used as desired.” Linn asserts that this statement does not suggest that any polymer percentage is acceptable but oppositely explicitly identifies the essential desired characteristics possessed by the films of the claimed invention and identifies the polymer levels needed to impart the characteristics.
As to the majority’s treatment of In re Werthiem, Linn notes that the majority cites no authority that written description support for a “closed range” requires a disclosure of a closed range rather than discrete values, and there is no logical reason why such a disclosure should be required as a strict rule to show possession. He remarks that in Wertheim, the CAFC had found “[b]roadly articulated rules are particularly inappropriate in this area.” Wertheim, 541 F.2d at 263-65 (Rich, J.) and an obvious example would be a disclosure with express embodiments of 5%, 6%, 7%, 8%, 9% and 10% of a particular substance, and a continuation application that claims a range of 5-10%. Relating this back to the discussion as to the disclosures of paragraph 65 of the application, he asserts that the paragraph does disclose a closed range of “at least 25%” and “at least 50%” and in light of In re Werthiem, those ranges are no different than if restated as “25%-100%” and “50%-100%,” respectively.
Hence, Linn concludes that he would reverse the Board’s holding that claims 1, 7 and 12 do not have written description support in the application and are thus anticipated by the prior art. Linn did concur-in-part as to the majority finding that Indivior was in possession of a film with 48.2 wt % polymeric matrix as claimed in claim 8.
Takeaway
- Care needs to be taken when patent prosecutors incorporate range limitations into the claims based on disclosures within Examples and Tables. The application should clearly contain evidence that the individual Examples may be formulated into the range claimed.
No Standing and No Vacatur for Patent Licensee Seeking Review of IPR Decisions
Fumika Ogawa | December 28, 2021
Apple Inc. v. Qualcomm Incorporated
Decided on November 10, 2021
Newman, Prost, and Stoll. Opinion by Prost. Dissent by Newman.
Summary
For a second time involving the same parties who had entered a license agreement as part of settlement of global patent litigation, the Federal Circuit denied standing to appeal from the Patent Trial and Appeal Board (PTAB) where the underlying facts were identical to those in the previous case, except for the identity of patents at issue. The Federal Circuit also rejected the licensee’s request to vacate the PTAB decision which was found unappealable for lack of standing. The dissent argued that the mere existence of a license should not negate the licensee’s right to challenge the patent validity in Article III court.
Details
“Flashback” to Apple I
The Opinion begins by referring to Apple Inc. v. Qualcomm Inc., 992 F.3d 1378, 1385 (Fed. Cir. 2021) (“Apple I”), where Qualcomm sued Apple in a district court for infringement of patents; Apple sought an inter partes review (“IPR”) of those patents; the two companies settled all their litigation and entered a license agreement, leading to dismissal of the infringement action with prejudice; thereafter, Apple appealed a final decision of the IPR issued in favor of Qualcomm.
At issue in Apple I was Apple’s standing before the Article III court. There, Apple was found to lack standing because:
- No sufficient evidence or argument was presented that invalidity of any specific patent would change any aspect of the contractual relationship or royalty imposed on Apple.
- Apple’s evidence failed to identify any particular patent or potentially infringing activity that is tied to a risk of litigation after the license has expired.
- Apple’s invocation of the estoppel provision was insufficient to warrant standing.
Rehearing was denied en banc in Apple I.
No Standing in Deference to Apple I
The Federal Circuit dismissed for lack of standing following the precedent. Since “the operative facts are the same,” the difference in the patents between the two cases was “irrelevant.” Further, the court rejected Apple’s assertion that the previous case did not articulate the reason why a threat of litigation that would potentially result from Apple’s failure to pay the license fee and termination of the agreement does not suffice to establish standing.
Vacatur Denied
Apple asserted that if it lacks standing, the PTAB decisions should be vacated, which would otherwise frustrate future litigation involving the same patents. In so doing, Apple relied on the principle in United States v. Munsingwear, Inc., which allows vacatur of a judgement below where the case has become moot on appeal.
The Federal Circuit disagreed. First, Munsignwear was distinguished because it concerns mootness, rather than standing. The difference between the two doctrines resides in the timing: Standing relates to existence of controversy “at the outset” of the appeal whereas mootness considers existence of controversy “throughout the proceedings.” To the extent the dispute between the parties had disappeared before the appeal was filed, the case cannot “become moot.” Second, even if the mootness doctrine were applicable, the court stated that vacatur would still not be appropriate. Because the alleged mootness was caused by Apple’s own voluntary action (i.e., settlement), it could not claim the equitable remedy of vacatur.
Dissent
The dissenting opinion noted that continuing controversy existed where Apple, although agreeing to settlement and license, still disputed the validity of the licensed patents, and the potentially infringing products will likely remain on the market after the termination of the contract, which does not cover the entire life of the patents as a result of Qualcomm’s refusal of Apple’s request otherwise. The dissent also argued that denial of the standing is contrary to the statutory purposes of estoppel and right of appeal provisions under the AIA. Furthermore, citing United States v. Arthrex, Inc., 141 S. Ct. 1970 (2021) (see Cindy Chen’s previous report), which required vacatur of PTAB decisions that are unreviewable by a principle agency officer, the dissent argued that the IPR decisions in the present case should be vacated if Apple is denied the constitutional right of judicial review.
Takeaway
- In addition to confirming that the patent licensee seeking appeal from the IPR lacks Article III standing in the circumstances of the case, the Federal Circuit found that such a party also forfeited its right to vacatur of the underlying IPR decision.
- Potential consequences of settlement and license where an IPR is pending could be grave; not only could it limit the party’s ability to prove a requisite injury for standing to appeal, but also it could foreclose the remedy of vacatur, thereby affecting future re-litigation.