Detecting Natural Phenomena Using Conventional Techniques Found “Directed to” Natural Phenomena at Alice/Mayo Step One
| September 16, 2022
CAREDX, INC. v. NATERA, INC.
Lourie, Bryson, and Hughes. Opinion by Lourie.
Summary
The CAFC held that genetic diagnostic method claims are ineligible for patent, finding that the claims reciting conventional laboratory techniques to perform diagnosis using a naturally occurring correlation are directed to natural phenomena under Alice/Mayo step one, and also lack additional elements to constitute enough inventive concept under Alice/Mayo step two.
Details
CareDx sued Natera and Eurofins in the U.S. District Court for the District of Delaware, asserting that their products infringed one or more patents licensed to CareDx. The district court awarded summary judgment for the defendants, holding that the patents are ineligible for patent under 35 U.S.C. §101. CareDx appealed the district court’s grant of the summary judgment motions of ineligibility.
The patents at issue, U.S. Patents 8,703,652, 9,845,497, and 10,329,607, relate to diagnosis of organ transplant status by detecting a donor’s cell-free DNA (“cfDNA”) circulating in a recipient’s body. The specification, common to all three patents, depicts prior findings that the existence of cfDNA in blood is mostly attributed to dead cells, and had been used for various diagnostic purposes, such as cancer diagnostics and prenatal testing. The specification notes that the cfDNA-based diagnostic scheme is applicable to organ transplant situations, where the recipient’s immune system kills incompatible donor’s cells which in turn release their nucleic acids into the recipient’s stream, such that an increased level of the donor-derived cfDNA may allow for detection of the transplant rejection.
Claim 1 of ‘652 patent recites:
1. A method for detecting transplant rejection, graft dysfunction, or organ failure, the method comprising:
(a) providing a sample comprising cell-free nucleic acids from a subject who has received a transplant from a donor;
(b) obtaining a genotype of donor-specific polymorphisms or a genotype of subject-specific polymorphisms, or obtaining both a genotype of donor-specific polymorphisms and subject-specific polymorphisms, to establish a polymorphism profile for detecting donor cell-free nucleic acids, wherein at least one single nucleotide polymorphism (SNP) is homozygous for the subject if the genotype comprises subject-specific polymorphisms comprising SNPs;
(c) multiplex sequencing of the cell-free nucleic acids in the sample followed by analysis of the sequencing results using the polymorphism profile to detect donor cell-free nucleic acids and subject cell-free nucleic acids; and
(d) diagnosing, predicting, or monitoring a transplant status or outcome of the subject who has received the transplant by determining a quantity of the donor cell-free nucleic acids based on the detection of the donor cell-free nucleic acids and subject cell-free nucleic acids by the multiplexed sequencing, wherein an increase in the quantity of the donor cell-free nucleic acids over time is indicative of transplant rejection, graft dysfunction or organ failure, and wherein sensitivity of the method is greater than 56% compared to sensitivity of current surveillance methods for cardiac allograft vasculopathy (CAV).
The representative claims of the three patents recite somewhat similar procedures, which may be summarized as:
- collecting a bodily sample from the recipient,
- “genotyping” or identifying genetic features that allow for distinction between the donor and recipient,
- “sequencing” or determining the makeup of cfDNA included in the sample, and
- determining, using the genetic features, the amount of cfDNA originating from the donor in the sample.
The specification depicts that the methods are performed using specific techniques that are “standard,” “well-established” and/or reported in prior patents and scientific articles, including sophisticated polymerase chain reaction (“PCR”), such as digital PCR and selective amplification, and next-generation sequencing (“NGS”), all of which are advanced, but already known, techniques in the field.
The district court held that that the asserted claims were patent ineligible as they were “directed to the detection of natural phenomena, specifically, the presence of donor cfDNA in a transplant recipient and the correlation between donor cfDNA and transplant rejection” and also, “recited only conventional techniques.”
On appeal, the CAFC performed the two-step Alice/Mayo analysis to determine patent-eligibility.
- Are the claims “directed to” laws of nature or natural phenomena? – Yes.
CareDx sought to characterize the claimed invention as directed to “improved measurement methods,” in particular, patent-eligible “use of digital PCR, NGS, and selective amplification” allowing for improved accuracy in cfDNA measurement, as opposed to “discovery of a natural correlation between organ rejection and the donor’s cfDNA levels in the recipient’s blood.” CareDx also asserted that the district court improperly considered conventionality of the claimed techniques at step one, essentially merging the two steps into a single-step analysis centered on conventionality.
The CAFC found that the claims satisfy the step one. Two contrasting precedents are notable: Illumina, Inc. v. Ariosa Diagnostics, Inc., 952 F.3d 1367, opinion modified by 967 F.3d 1319, 1327 (Fed. Cir. 2020), and Ariosa Diagnostics, Inc. v. Sequenom, Inc., 788 F.3d 1371 (Fed. Cir. 2015). The CAFC noted that this case is different from Illumina, where the claimed improvement was a patent-eligible “method for preparing” a cfDNA fraction that would not occur naturally without manipulation of a starting sample; rather, the asserted claims are akin to those in Ariosa, wherein the claimed diagnostic methods, including the steps of “amplifying” (i.e., making many copies of) a cfDNA sample using PCR and “detecting” a certain type of cfDNA, so as to perform diagnosis using a natural correlation between certain conditions and the level of cfDNA, were found to be “directed to a natural phenomenon.”
The CAFC noted that the conventionally considerations are not limited to step two, and precedents have routinely performed overlapping conventionality inquiry at both stages of Alice/Mayo. The CAFC found that the use of specific laboratory techniques relied on by CareDx only amounts to “conventional use of existing techniques to detect naturally occurring cfDNA.” The CAFC added that the conventionality is supported by the specification’s numerous remarks characterizing the claimed techniques as “any suitable method known in the art” and similar boilerplate language.
- Do the claims recite additional elements, aside from the natural phenomena, which transform the nature of the claim’ into a patent-eligible application? – No.
CareDx’s main argument at step two was that the inventive concept resides in the use of the specific advanced techniques to identify and measure donor-derived cfDNA.
The CAFC disagreed, concluding that the claimed methods lack requisite inventive concept. In reaching the conclusion, the CAFC again pointed to the specification’s admissions of the conventionality of the individual techniques recited in the claims. The CAFC went on to state that “[t]he specification confirms that the claimed combination of steps … was a straightforward, logical, and conventional method for detecting cfDNA previously used in other contexts,” which adds nothing inventive to the detection of natural phenomena.
Takeaway
This case provides a reminder that conventionality of claimed elements may affect both steps of Alice/Mayo test. At step one, the effort to characterize the claim as being “directed to” a patent-eligible subject matter can be thwarted where the claimed elements are undisputedly conventional, in the absence of an unconventional element that is not a judicial exception to eligibility. And at step two, the conventionality of the individual elements and their combination prohibits finding of inventive concept.
When does the absence of evidence turn into evidence of absence? The CAFC vacate their prior decision to now hold that there must be evidence that a skilled artisan would understand silence regarding a limitation to necessarily exclude said limitation.
| September 9, 2022
Novartis Pharmaceuticals v. HEC Pharms Co, Ltd, & Accord Health Care et al.
Summary:
HEC petitioned for rehearing of a CAFC prior decision in this case, (21 F 4th 1362 – Fed. Cir. 2022) in which the CAFC affirmed a final judgement of the Delaware district court determining that claims 1 to 6 of US Patent 9,187,405 are not invalid and that HEC infringes them. The CAFC Panel in the prior case was Judges, Moore (dissent) with Linn and O’Malley. Here, Chief Judge Moore with Circuit Judge Hughes (majority herein after) vacate the CAFC’s prior decision holding that the Novartis claims are invalid for inadequate written description pertaining to a negative limitation.
- Background
Novartis owns the ‘405 patents and markets a drug for treating relapsing remitting multiple sclerosis that purportedly practices the patent. HEC filed an ANDA with the FDA seeking approval to market a generic version of the drug. Novartis sued. The Delaware district count found that the claims were not invalid either as anticipated or for inadequate written description of the no-loading-dose or daily dosage limitations. HEC appeals as to the written description issue of the no-loading dose.
Claim 1 at issue recites in part “a daily dosage of 0.5 mg, absent an immediately preceding loading dose regime.” The appeal at hand being related to the underlined negative limitation. A loading dose is a higher-than-normal daily dose.
The Majority go into detail regarding what is required to satisfy the written description, stating that “[F]or negative claim limitations…. there is adequate written description when., for example, “the specification described a reason to exclude the relevant [element]… A reason to exclude an element could be found in ‘statements in the specification expressly listing the disadvantages of using’ that element and alternatives to it…. Silence is generally not disclosure.” The Majority further noted that the negative limitation may not be recited verbatim in the specification, but there “generally must be something in the specification that conveys to a skilled artisan that the inventor intended the exclusion, such as a discussion of disadvantages or alternatives.” However, the Majority further noted that while “a written descriptions silence about a negative claim limitation is a useful and important clue and may often be dispositive, it is possible that the written description requirement may be satisfied when a skilled artisan would understand the specification as inherently disclaiming the negative limitation.”
The district count had found that the negative limitation was supported because the prophetic trial described therein states “initially” giving a daily dose, thus this would inform the skilled artisan that there was no loading dose. The Majority found this interpretation erroneous arguing that because the specification says “Initially patients receive treatment for 2 to 6 months” it is clear this sentence speaks to the initial length of treatment and not the dosage. The Majority believed that one of the two Novartis experts admitted this, and that the contrary testimony by the second Novartis experts was inconsistent with the plain text of the specification and therefore carried no weight.
Next, the Majority discussed the district courts finding that the specifications disclosure of a daily dosage combined with its silence regarding a loading dose would tell a person of skill that loading doses are excluded. However, the Majority countered that a patent is not presumed complete such that things not mentioned are necessarily excluded. The Majority noted that the applicants added the limitation during prosecution to address a prior art rejection arguing that the limitation was “to specify that the [daily dosage] cannot immediately follow a loading dose regiment” and “to further distinguish their claims.” The Majority argued that if reciting “daily dosage” without mentioning a loading dose necessarily excludes said loading dose, there would have been no reason for the applicants to add the limitation.
The Majority noted that the expert testimony focused on where in the specification the patentee would have mentioned a loading dose if they intended a loading dose to be included, but that is not the issue at hand, rather the question is whether the patentee precluded the use of a loading dose. The Majority concluded that there is no evidence that a skilled artisan would understand silence regarding a loading artisan to necessarily exclude a loading dose.
Thus, the Majority vacated their prior decision and reversed the distinct courts judgement that the claims of the ‘405 patent are not invalid.
- Dissent:
Circuit Judge Linn dissented, her opinion being very reminiscent of her opinion in the prior judgment, for which she penned the Majority. CJ Linn stated that the “majority in its analysis employs the heightened standard of “necessary exclusion” against which to assess the district court’s fact findings in this case and uses that standard to conclude that the district court clearly erred.” CJ Linn argued that the central tenet of the written description jurisprudence is that the disclosure must be read from the perspective of a person of skill in the art. Here, CJ Lin argued that the district conducted an objective inquiry into the four corners of the specification and found sufficient written description, while also crediting the expert testimony. In particular, the testimony that one skilled in the art would understand the loading dose to be excluded, and that loading dose regiments have been used in the prior art for treating MS. CJ Linn concluded this is sufficient to show that claim language that precludes the administration of a loading dose is supported.
Take-away:
- When express support for a negative limitation is absent, look for evidence that a skilled artisan would understand the silence regarding the limitation to necessarily exclude said limitation.
- Be mindful of statements made during prosecution as to why an amendment is being made.
CAFC HOLDS THAT A SIMPLE ERROR IS STILL SIMPLE EVEN IF IT IS HARD TO FIND
| August 23, 2022
LG Electronics Inc. v. Immervision, Inc.
NEWMAN, STOLL, and CUNNINGHAM. Opinion by Stroll. Dissent by Newman.
Summary:
CAFC affirms PTAB that LG failed to demonstrate obviousness in its IPR petitions relying on a “copy-and-paste” error which took an expert detailed analysis to locate. The Court and Board both found the error to be within the Yale standard that an obvious error of a typographical or similar nature that would be apparent to one of ordinary skill in the art who would mentally disregard the errant information as a misprint or mentally substitute it for the correct information, the errant information cannot be said to disclose subject matter.
Background:
LG Electronics Inc. appealed from the United States Patent Trial and Appeal Board’s final written decisions in a pair of inter partes review proceedings challenging claims 5 and 21 of U.S. Patent No. 6,844,990 asserted by Immervision.
The ’990 patent specification describes capturing an initial digital panoramic image using an objective lens having a non-linear image point distribution function that “expands certain zones of the image and compresses other zones of the image.” Id. at col. 3 l. 62–col. 4 l. 38. The “non-linearity of the initial image” can then be corrected to produce a final panoramic image for display. Id. at col. 4 ll. 47–53. “[T]he expanded zones of the image cover” a higher “number of pixels of the image sensor” than they would with a lens having linear image point distribution. Id. at col. 3 l. 62–col. 4 l. 10. The exemplary claim used by the Court was claim 5:
5. The method according to claim 1, wherein the objective lens compresses the center of the image and the edges of the image and expands an intermediate zone of the image located between the center and the edges of the image.
LG filed two petitions for inter partes review, each challenging a dependent claim of the ’990 patent. J.A. 322–66 (IPR2020-00179 challenging claim 5); J.A. 3338–87 (IPR2020-00195 challenging claim 21). In the petitions LG argued that U.S. Patent No. 5,861,999 (“Tada”), directed to a “Super Wide Angle Lens System Using an Aspherical Lens.”2 Tada describes four embodiments that share a general system structure and differ in aspects such as lens element thick-ness, separation distance, and lens shape. The embodiment relevant to the appeal, Embodiment 3, described by a prescription—or set of optical parameters—set forth in Table 5 of its specification.
LG argued that Tada discloses, as recited in the challenged claims, a panoramic objective lens having a non-linear image point distribution that compresses the center and edges of an image and expands an intermediate zone of the image between the center and the edges of the image. Tada, however, does not explicitly discuss the image point distribution functions of its lenses. Instead, LG relied on its expert Dr. Chipman’s declaration for the proposition that Tada’s third embodiment has a distribution function producing “a compressed center and edges of the image and an expanded intermediate zone of the image between the center and the edges of the image” as recited in challenged claims 5 and 21.
Dr. Chipman “reconstruct[ed] the lens of Figure 11 [of Tada] using the information in Table 5 of Tada” by inputting certain “information from Table 5 [as published] . . . into an optical design program.” J.A. 1486–87. LG relied exclusively on Dr. Chipman’s calculations and plots using the prescription in Table 5 to show that Tada’s third embodiment meets the compression and expansion zone limitation of the challenged claims.
In its patent owner response, ImmerVision, relied on its expert witness Mr. Aikens’ declaration, arguing that Tada’s Table 5 includes a readily apparent error that cannot form the basis of any obviousness ground. Specifically, Mr. Aikens first noticed that the physical surface of his lens model based on Tada’s Table 5 and the example lens depicted in Tada’s Figure 11 did not match. Because of this discrepancy, Mr. Aikens compared the sag table—a table of heights of a lens surface with respect to the optical axis—generated for his lens model with the sag table provided in Tada’s Table 6 corresponding to Embodiment 3. They also did not match. After Mr. Aiken’s extensive review which included comparing the ‘990 patent with its priority Japanese application, it became apparent that there was a transcription, or copy-and-paste, error in Tada. The disclosures in Tada’s Table 5, which were intended to correspond to its Embodiment 3, were actually identical to those in Table 3, which corresponded to Embodiment 2.
Based on Mr. Aiken’s Declarations, the Board found that the “disclosure of aspheric[] coefficients in Table 5 of Tada is an obvious error” that a person of ordinary skill in the art would have recognized and corrected. Further, the Board found that because the correct aspheric coefficients in Table 5 of the Japanese Priority Application do not satisfy the language of the challenged claims, LG had not met its burden to prove the challenged claims unpatentable as obvious. The Board concluded that LG did not meet its burden to prove the challenged claims would have been obvious by a preponderance of evidence.
LG appealed.
Decision:
First, the Court noted that it is undisputed that the aspheric coefficients in Tada’s Table 5 were erroneous. Therefore, their decision focused on whether substantial evidence supports the Board’s fact finding that the error would have been apparent to a person of ordinary skill in the art such that the person would have disregarded the disclosure or corrected the error.
Second, the Court set forth the legal standard for such a review, citing to In re Yale, 434 F.2d 666 (C.C.P.A. 1970), as the controlling case law. They noted that Yale held that where a prior art reference includes an obvious error of a typographical or similar nature that would be apparent to one of ordinary skill in the art who would mentally disregard the errant information as a misprint or mentally substitute it for the correct information, the errant information cannot be said to disclose subject matter. Id. at 669.
Next, the Court reviewed the Board’s fact finding. They affirmed that the Board correctly identified several aspects of the disclosure in Table 5 that would alert the ordinarily skilled artisan that the disclosure was an obvious error of a typographical or similar nature. Specifically, they noted that it was undisputed that Tada’s Tables 5 and 9 are inconsistent: the aspheric coefficients A4, A6, and A8 in Tada’s Table 5 should match the values for conditions (2)–(4) in Table 9 but do not. They contrasted this to the facts in Yale, finding precedent (describing the internal inconsistency within a reference as a signal that a person of ordinary skill “would readily recognize” as portending error). Yale, 434 F.2d at 667. Based thereon, the Court concluded that
Table 5 cannot be said to disclose a lens that compresses the center of the image and the edges of the image and expands an intermediate zone of the image located between the center and the edges of the image as required by claim 5 of the ‘990 patent.
The CAFC also reviewed LG’s arguments attempting to distinguish the current fact pattern from Yale. First, LG contended that Yale sets forth an “Immediately Disregard or Correct” standard that imposes a temporal urgency on the discovery of the error before the error can be considered “obvious” to a skilled artisan. Applying this reading of Yale, LG argued that Mr. Aikens’ “convoluted process” that took “ten to twelve hours” to complete clearly weighed against the obviousness of the error. LG asserted that because Tada remained uncorrected in the public domain for over 20 years, LG should have been able to rely on the aspheric coefficients in Tada’s Table 5 as published.
In response, the Court held that LG’s suggestion that Yale requires a person of ordinary skill in the art to immediately recognize the apparent error is incorrect. The Court affirmed the PTAB’s holding that the length of time and the “particular manner” in which the error was actually discovered “does not diminish that there is an obvious error in Tada within the meaning of Yale.”
Contrary to LG’s assertions, Yale does not impose a temporal requirement. Nor does LG cite any other authority requiring that the error be discovered within a specified amount of time. Certainly, the amount of time it takes a skilled artisan to detect an error may be relevant to whether an error is, in fact, an apparent error under Yale. But this is just one factor for the fact finder to consider as part of the overall analysis. Here, the Board considered the totality of circumstances and found that Tada’s disclosure of aspheric coefficients in Table 5 is an obvious error of a typographical or similar nature, notwithstanding the amount of time that preceded detection of the obvious error.
Second, LG argued that Yale is limited to instances in which the error is a typographical error, and therefore should be narrowly limited to errors such as spelling mistakes and similar minor, easily detectable errors. The CAFC disagreed, finding instead that while the error in Yale was typographical, the error at issue here was not so far afield as to warrant a different outcome.
The distinction between the typographical error in Yale and the copy-and-paste error here is a distinction without a difference.
Based thereon, the Court affirmed the PTAB’s ruling that LG failed to demonstrate obviousness based on the erroneous calculations set forth in the Tada reference.
Dissent
Judge Newman dissented, asserting that she could not agree that the error in the Tada Tables was typographical or similar in nature, because its existence was not discovered until an expert witness conducted a dozen hours of experimentation and calculation.
She asserted that the appearance of a few of the same numbers in two different tables in the Tada reference provides no information as to which numbers and tables are correct and which may be in error. Contrary, she considers a typographical or similar error needs to be apparent to the reader and may conveniently be ignored without impeaching the content of the information.
Judge Newman outlines the extensive history of the ‘990 patent, noting that no one detected the error until Immervision’s expert spent significant time ascertaining that an error was in fact present in the ‘990 patent. She asserts that these events that preceded the expert’s discovery of the error in the Tada reference cannot be ignored.
The specifics of what led the expert, Mr. Aikens, to discover the erroneous values in Table 5 also cast doubt on whether the error may be deemed “obvious and apparent.” Mr. Aikens testified that he had fully modeled Tada’s Embodiment 3—relying on data from Table 5—without noticing the error. … It was only after his model was completed that he noticed the lens created a distorted image, leading him to presume there was perhaps some error in Tada.
The Japanese application had the correct aspherical values in Table 5, as confirmed by a skilled expert in this technology, after many hours of corrective effort that included fully modeling three separate embodiments of the lens. In sum, the error was not of “typographical or similar nature.”
Judge Newman contrasts this to the evidence in Yale, noting that the error therein did not require calculations or experimentation, and that without the Japanese Priority Application, there is no source of the correct information in the current case.
She agreed with the panel majority that Yale establishes the correct standard to determine if an error would be obvious to a person of ordinary skill in the field, but maintains that an “obvious error” should be apparent on its face and should not require the conduct of experiments or a search for possibly conflicting information to determine whether error exists.
Take away:
Relying on an error in prior art as teaching aspects of a claim is less likely to be sufficient to establish that the subject matter was known even if the error is difficult to locate. Care needs to be taken in examining the prior art reference including contrasting to the disclosures of priority documents. The Yale standard of whether an error can be relied upon looks to the simplicity of the error not to the scrutiny necessary to locate it.
General industry skepticism may not be sufficient by itself to preclude a finding of motivation to combine
| August 2, 2022
Auris Health, Inc. v. Intuitive Surgical Operations, Inc.
Decided: April 29, 2022
Prost (author), Dyke, and Reyna (dissenting)
Summary:
The Federal Circuit rejected the PTAB’s obviousness decision by holding that general industry skepticism is not relevant to the question of obviousness.
Details:
In 2018, Intuitive Surgical sued Auris Health for patent infringement of U.S. Patent No. 8,142,447 (“the ’447 patent”).
The ’447 patent
This patent is directed to robotic surgery systems.
The ’447 patent describes “an improvement over Intuitive’s earlier robotic surgery systems, which allow surgeons to remotely manipulate surgical tools using a controller.”
The ’447 patent attempts to solve problems (doctors must swap out various surgical instruments and this step could be tricky in a robotic surgical system where space is limited, different ranges of motion must be calibrated for different surgical instruments, and time is need to interchange those instruments) in the surgery via a robotic system with a servo-pulley mechanism so that doctors could swap out surgical instruments and reduce surgery time, improve safety, and increase reliability of the system.
PTAB
The PTAB determined that the prior art references asserted by Aurie – Smith and Faraz – disclosed each limitation of the claims in the ’447 patent.
The only remaining issue is whether a skilled artisan would have motivated to combine these two references.
Smith discloses “a robotic surgical system that uses an exoskeleton controller, worn by a clinician, to remotely manipulate a pair of robotic arms, each of which holds a surgical instrument.” Smith discloses that the clinician may direct an assistant to relocate the arms as necessary.
Faraz is directed an adjustable support stand that holds surgical instruments. Faraz discloses that its stand “may enable a surgeon to perform surgery with fewer assistants” because its stand “can support multiple surgical implements while [they] are being moved” and “can also provide support for a surgeon’s arms during long or complicated surgery.”
Auris argued that a skilled artisan would be motivated to combine these references to decrease the number of assistants necessary for surgery.
Intuitive argued that a skilled artisan would not be motivated to combine these references because “surgeons were skeptical about performing robotic surgery in the first place, [so] there would have been no reason to further complicate Smith’s already complex robotic surgical system with [Faraz’s] roboticized surgical stand.”
The PTAB agreed with Intuitive that there is no motivation to combine these references because there is skepticism at the time of the invention for using robotic systems during surgery in the first place.
Federal Circuit
The Federal Circuit held that general industry skepticism cannot, by itself, preclude a finding of motivation to combine.
The Federal Circuit noted that the evidence of skepticism must be specific to the invention, not generic to the field and that Intuitive provided not case law to suggest that the PTAB can rely on generic industry skepticism to find a lack of motivation of combine.
The Federal Circuit held that the PTAB was wrong to exclusively rely on Intuitive’s expert testimony that “there was great skepticism for performing telesurgery” at the time of the invention and, as a result, a skilled artisan “would not have been compelled to complicate Smith’s system further.”
Therefore, the Federal Circuit remand for further consideration of the parties’ motivation-to-combine evidence.
Dissent
Judge Reyna noted that the PTAB’s determination that Auris failed to show a motivation to combine is adequately supported by substantial evidence and was not contrary to our law on obviousness.
While Judge Reyna agreed that skilled artisans’ general skepticism toward robotic surgery, by itself, could be insufficient to negate a motivation to combine, he disagreed that it could never support a finding of no motivation to combine.
Judge Reyna believes that the majority’s inflexible and rigid rule appears to be in tension with the central thrust of KSR (rejecting the “rigid approach of the Court of Appeals” and articulating an “expansive and flexible approach” of determining obviousness).
Takeaway:
- General industry skepticism may not be sufficient by itself to preclude a finding of motivation to combine.
- Specific evidence of industry skepticism related to a specific combination of references might contribute to finding a lack of motivation to combine.
The applicants’ statement during the prosecution may play a critical role for claim construction
| July 4, 2022
Sound View Innovations, LLC v. Hulu, LLC
Decided: May 11, 2022
Prost, Mayer (Sr.) and Taranto. Court opinion by Taranto.
Summary
On appeals from the district court for summary judgment of noninfringement of a patent, directed to a method of reducing latency in a network having a content server, the Federal Circuit affirmed the district court’s construction of the down-loading/retrieving limitation solely on the basis of prosecution history, but the Court rejected the district court’s determination that “buffer” cannot cover “a cache,” and vacated the district court’s grant of summary judgment and remanded for further proceedings.
Details
I. Background
(i) The Patent in Dispute
Sound View Innovations, LLC (“Sound View”) owns now-expired U.S. Patent No. 6,708,213 (“the ’213 patent”), which describes and claims “methods which improve the caching of streaming multimedia data (e.g., audio and video data) from a content provider over a network to a client’s computer.” Claim 16 of the ’213 patent provides as follows:
16. A method of reducing latency in a network having a content server which hosts streaming media (SM) objects which comprise a plurality of time-ordered segments for distribution over said network through a plurality of helpers (HSs) to a plurality of clients, said method comprising:
receiving a request for an SM object from one of said plurality of clients at one of said plurality of helper servers;
allocating a buffer at one of said plurality of HSs to cache at least a portion of said requested SM object;
downloading said portion of said requested SM object to said requesting client, while concurrently retrieving a remaining portion of said requested SM object from one of another HS and said content server (the “downloading/retrieving limitation”); and
adjusting a data transfer rate at said one of said plurality of HSs for transferring data from said one of said plurality of helper servers to said one of said plurality of clients (emphasis added).
During the prosecution, the examiner rejected original claim 16—which was identical to issued claim 16 except that it lacked the downloading/retrieving limitation—as anticipated over DeMoney (U.S. Patent No. 6,438,630). To overcome the rejection, the applicants amended the claim to add the downloading/retrieving limitation. The applicants explained that support for the added limitation is found in the applicants’ specification, pointing to the portion of the specification that shows concurrent downloading and retrieval involving a single buffer (emphasis added). In fact, the specification nowhere says that the invention includes use of separate buffers for the concurrent downloading and retrieving functions, and it nowhere illustrates or describes such an embodiment, in which different buffers are involved in con- current downloading of one portion and retrieving of a remaining portion of the same SM object in response to a given client’s request.
(ii) The District Court
Sound View sued Hulu, LLC (“Hulu”) for infringement of six Sound View patents including the ’213 patent in the United States District Court for the Central District of California (“the district court”). As the case proceeded, only claim 16 of the ’213 patent remained at issue.
It was undisputed that the accused edge servers— the edge servers Sound View identified as the “helper servers” for its infringement charge—do NOT download and retrieve subsequent portions of the same SM object in the same buffer (emphasis added).
Sound View alleged, among other things, that, under Hulu’s direction, when an edge server receives a client request for a video not already fully in the edge server’s possession, and obtains segments of the video seriatim from the content server (or another edge server), the edge server transmits to the Hulu client a segment it has obtained while concurrently retrieving a remaining segment.
The district court agreed with Hulu’s argument that the applicants’ statements accompanying the amendment in the prosecution disclaimed the full scope of the downloading/retrieving limitation, and that the downloading/retrieving limitation thus required that the same buffer in the helper server—the one allocated in the preceding step—host both the portion sent to the client and a remaining portion retrieved concurrently from the content server or other helper server (emphasis added).
In reliance on the construction, Hulu then sought summary judgment of non-infringement of claim 16, arguing that it was undisputed that, in the edge servers of its content delivery networks, no single buffer hosts both the video portion downloaded to the client and the retrieved additional portion. Sound View countered that there remained a factual dispute about whether “caches” in the edge servers met the concurrency limitation as construed. The district court held, however, that a “cache” could not be the “buffer” that its construction of the down- loading/retrieving limitation required, and on that basis, it granted summary judgment of non-infringement. A final judgment followed.
Sound View timely appealed.
II. The Federal Circuit
The Federal Circuit (“the Court”) affirmed the district court’s construction of the down-loading/retrieving limitation. but the Court rejected the district court’s determination that “buffer” cannot cover “a cache,” and vacated the district court’s grant of summary judgment and remanded for further proceedings.
Claim Construction (the downloading/retrieving limitation)
The Court first noted that this is not a case in which the other intrinsic evidence—the claim language and specification—establish a truly plain meaning contrary to the meaning assertedly established by the prosecution history because the downloading/retrieving limitation, which does not expressly refer to “buffers,” contains no words affirmatively making clear that different buffers in the helper server may be used for the sending out to clients of one portion of the SM object and the receiving of a retrieved remaining portion.
The Court viewed the prosecution history as establishing that the applicants made clear that the applicants’ invention concurrently empties and fills the buffer, while the DeMoney reference teaches filling the buffer only after the buffer is empty,” citing column 12, lines 28–40 of DeMoney (emphasis added).
Based on the applicants’ statements, the Court agreed with the district court that the applicants limited claim 16 to using the same buffer for the required concurrent downloading and retrieval of portions of a requested SM object.
Summary Judgment of Non-Infringement
After the district court adopted its “buffer”-requiring claim construction of the downloading/retrieving limitation, it granted summary judgment of non-infringement, concluding that accused-system components called “caches”—on which Sound View relied for its allegation of infringement under the court’s claim construction—could not be the required “buffers.” The district court relied for its conclusion on the ’213 patent’s references to its described “buffers” and “caches” as distinct physical components. To support the conclusion, the district court noted that the ’213 patent describes buffers and caches in different locations: The patent defines “cache” as “a region on the computer disk that holds a subset of a larger collection of data,” and although the patent does not define “buffer,” it describes “ring buffers” located “in the memory” of the HS.
However, the Court stated that the district court’s construction here was inadequate for the second step of an infringement analysis (comparison to the accused products or methods) because it did not adopt an affirmative construction of what constitutes a “buffer” in this patent. More specifically, the Court stated that the district court did not decide, and the record does not establish, that “cache” is a term of such uniform meaning in the art that its meaning in the ’213 patent must be relevantly identical to its meaning when used by those who labeled the pertinent components of the accused edge servers. The Court thus concluded that, in the absence of such a uniformity-of-meaning determination, the district court’s conclusion that the ’213 patent distinguishes its buffers and caches is insufficient to support a determination that the accused-component “caches” are outside the “buffers” of the ’213 patent. What was needed was an affirmative construction of “buffer”— which could then be compared to the accused-component “caches” based on more than a mere name.
For an additional reason for the further affirmative construction, the Court pointed out that, even in the ’213 patent, the terms “buffer” and “cache” do not appear to be mutually exclusive, but instead seem to have at least some overlap in their coverage given that the disputed claim describes “allocating a buffer . . . to cache” a portion of the SM object, and that the specification explains that “the ring buffer . . . operates as a type of short term cache” because it is capable of servicing multiple client requests within a certain time interval (emphasis added).
Takeaway
· Claim construction: The applicants’ statement during the prosecution may play a critical role for claim construction if the claim language and specification do not establish a truly plain meaning of a disputed term contrary to the meaning assertedly established by the prosecution history.
· Summary Judgment: Portions of the specification may provide genuine issue of material fact for denial of summary judgment (in this case, the embodiment describes buffers and caches in different locations while the disputed claim provides “allocating a buffer . . . to cache”)
Inventor’s Own Provisional Application Can Be Prior Art Against Inventor’s Own US National Stage Application
| June 10, 2022
Konda v. Flex Logix Technologies, Inc.
Decided: May 6, 2022
per curiam: Taranto, Clevenger, Chen (May 6, 2020).
Summary:
In Flex Logic’s inter partes reviews (IPRs), the PTAB agreed with Flex Logic in that the ‘605 PCT and the ‘394 Provisional application did not support the challenged claims of the ‘523 patent, and therefore, the ‘523 patent could not benefit from the earlier filing dates of the ‘605 PCT and the ‘394 Provisional application and that the ‘523 patent benefited only from its filing date of November 22, 2009. The PTAB agreed that a prior art ‘756 PCT publication (another one of Konda’s earlier patent applications published on September 12, 2008) and the ‘394 provisional application (that was incorporated by reference into the ‘756 PCT) rendered all the challenged claims unpatentable. The only issue on appeal was whether the ‘394 Provisional is publicly available to qualify as prior art. The Federal Circuit held that Konda’s US ‘394 Provisional application that is incorporated by reference in a publicly available publication of a PCT application is “publicly available” as prior art under pre-AIA 35 USC §102(b).
Background:
Konda’s US Patent No. 8,269,523 issued from US patent application s/n 12/601,275 (the ‘275 application) filed November 22, 2009. The ‘275 application is the national phase entry of PCT application no. PCT/US2008/064605 (the ‘605 PCT) filed May 22, 2008. The ‘275 application also claimed priority back to Provisional application no. 60/940,394 (the ‘394 Provisional) filed May 25, 2007. Flex Logic’s IPRs relied upon WO 2008/109756 (the ‘756 PCT) and the ‘394 Provisional as prior art against the challenged claims of the ‘523 patent. The ‘756 PCT is another one of Konda’s earlier patent applications that incorporated by reference the ‘394 Provisional. The ‘756 PCT was published on September 12, 2008.
Procedural History:
Two inter partes reviews (IPRs) were filed by Flex Logic, addressing different sets of claims in Konda’s US Patent No. 8,269,523 (the ‘523 patent). The PTAB’s Final Decision in the consolidated IPRs found that the ‘605 PCT and the ‘394 Provisional applications did not have support for the claims of the ‘523 patent as required by 35 USC §112. Accordingly, the ‘523 patent only benefited from its filing date of November 22, 2009. The PTAB also found that all the challenged claims to be unpatentable as being anticipated or obvious over the ‘756 PCT published on September 12, 2008 and the ‘394 provisional application that was incorporated by reference into the ‘756 PCT. The only issue on appeal to the Federal Circuit was whether the ‘394 Provisional was publicly available to qualify as prior art under pre-AIA 35 USC §102(b).
Relevant Statute and Rules:
35 U.S.C. 122(a):
(a) CONFIDENTIALITY.— Except as provided in subsection (b), applications for patents shall be kept in confidence by the Patent and Trademark Office and no information concerning the same given without authority of the applicant or owner unless necessary to carry out the provisions of an Act of Congress or in such special circumstances as may be determined by the Director
37 CFR 1.14(a)(1)(vi):
(vi) Unpublished pending applications (including provisional applications) that are incorporated by reference or otherwise identified. A copy of the application as originally filed of an unpublished pending application may be provided to any person, upon written request and payment of the appropriate fee (§ 1.19(b)), if the application is incorporated by reference or otherwise identified in a U.S. patent, a statutory invention registration, a U.S. patent application publication, an international publication of an international application under PCT Article 21(2), or a publication of an international registration under Hague Agreement Article 10(3) of an international design application designating the United States. The Office will not provide access to the paper file of a pending application, except as provided in paragraph (c) or (i) of this section.
37 CFR 1.14(c):
(c) Power to inspect a pending or abandoned application. Access to an application may be provided to any person if the application file is available, and the application contains written authority (e.g., a power to inspect) granting access to such person. The written authority must be signed by:
(1) The applicant;
(2) A patent practitioner of record;
(3) The assignee or an assignee of an undivided part interest;
(4) The inventor or a joint inventor; or
(5) A registered attorney or agent named in the papers accompanying the application papers filed under § 1.53 or the national stage documents filed under § 1.495, if a power of attorney has not been appointed under § 1.32.
Decision:
There is no dispute that the ‘756 PCT is an international patent application that incorporated by reference the ‘394 Provisional application, thereby meeting the requirements of 37 CFR 1.14(a)(1)(vi) for public accessibility to the ‘394 Provisional application.
Konda relied on the last sentence of 37 CFR 1.14(a)(1)(vi) whereby “The Office will not provide access to the paper file of a pending application, except as provided in paragraph (c) or (i) of this section.” And, Konda did not provide any written authority pursuant to 37 CFR 1.14(c) to provide access the ‘394 Provisional application. Konda also relied on MPEP 103(VII) (8th ed., rev. July 7, 2008) stating that access to provisional applications “will only be given to parties with written authority from a named inventor, the assignee of record, or the attorney or agent of record.”
However, the Federal Circuit noted the last sentence of 37 CFR 1.14(a)(1)(vi) pertains only to access to the “paper file” of the pending application, which the Federal Circuit interprets to mean the “whole file history,” which the regulation clearly distinguishes from the “application as originally filed.” It is the “application as originally filed” to which 37 CFR 1.14(a)(1)(vi) grants public accessibility when that “application as originally filed” was incorporated by reference in a PCT application. The last sentence of 37 CFR 1.14(a)(1)(vi) and 37 CFR 1.14(c) do not apply to the “application as originally filed.” There was no need for Konda’s written authorization to access that “application as originally filed.”
As for the MPEP, the court noted that the MPEP “does not have the force of law.” The court also noted that MPEP 103 further stated that provisional applications were “also available in the same manner as any other application” “thereby permitting the access authorized under 37 CFR 1.14(a)(1)(vi).”
Takeaways:
- If, following the inventor’s incorporation by reference preferences, the ‘605 PCT in the subject ‘523 patent family incorporated by reference the ‘756 PCT, perhaps 112 support could have been argued for the ‘523 claims. Nevertheless, as can be appreciated from the result of this case (an inventor’s own provisional application was used as prior art against his own national stage application!), applicants must be careful when incorporating by reference applications from a different patent application family.
- In the PTAB’s Final Decision, the entire 112 support discussion is premised on case law regarding entitlement to the benefit of the filing date of an earlier filed application only if the disclosure of the earlier filed application provides support for the claims of the later application. And, somehow, the national stage application of an international PCT application is only accorded the benefit of its “filing date” of the national stage ‘275 application. However, the international ‘605 PCT application is not “an earlier filed application” from the national stage application thereof. As noted in MPEP 1893.03(b):
An international application designating the U.S. has two stages (international and national) with the filing date being the same in both stages. Often the date of entry into the national stage is confused with the filing date. It should be borne in mind that the filing date of the international stage application is also the filing date for the national stage application.
Pursuant to 35 USC 363:
An international application designating the United States shall have the effect, from its international filing date under Article 11 of the treaty, of a national application for patent regularly filed in the Patent and Trademark Office.
Similarly, PCT Article 11(3) provides that…
an international filing date shall have the effect of a regular national application in each designated State as of the international filing date, which date shall be considered to be the actual filing date in each designated State.
Here, the ‘275 national stage application appears to be identical to the ‘605 PCT application. Nevertheless, this issue was never challenged before the PTAB nor the Federal Circuit.
Tags: Earliest effective date > Incorporation by reference > PCT application > Publicly available as prior art
TRANSACTIONS WITHOUT PRIMARY EXPERIMENTAL-USE PURPOSE DO NOT INSULATE FROM THE ON-SALE BAR
| June 3, 2022
Sunoco Partners Marketing & Terminals L.P., v. U.S. Venture, Inc., U.S. Oil Co., Inc.
Decided: April 29, 2022
Before PROST, REYNA, and STOLL, Circuit Judges.
Summary
The Federal Circuit affirmed in part, reversed in part, vacated in part, and remanded judgement of the United States District Court for the Northern District of Illinois regarding patent infringement. In one of the decisions, the Federal Circuit overturned a district court judgment on some of Sunoco’s claims insulating from the on-sale bar.
Background
Sunoco Partners Marketing & Terminals L.P. (“Sunoco”) sued U.S. Venture, Inc. and U.S. Oil Co., Inc. (collectively, “Venture”), alleging that Venture’s operation butane-blending systems infringed claims of U.S. Patent Nos. 7,032,629 (“the ’629 patent”), 6,679,302 (“the ’302 patent”), 9,494,948 (“the ’948 patent”), and 9,606,548 (“the ’548 patent”) owned by Sunoco.
The patents claimed systems and methods that are directed to blending butane into gasoline before the end chain of distribution. Butane blended with gasoline has some advantages: butane’s volatility helps cars start more easily at low temperatures, and butane is cheaper than gasoline. However, since butane causes air pollution when burned in warm climate, the Environmental Protection Agency (EPA) established regulations on butane blending. Sunoco’s patented technology seeks to maximize butane content while complying with EPA’s (U.S. Environmental Protection Agency) regulations.
The district court ruled on various summary judgment motions and sided with Sunoco in a bench trial to affirm the award of $2 million in damages, later trebled to $6 million.
Discussion
Venture appealed the district court’s decision on (I) rejection of its on-sale-bar defense, (II) determination that it infringed two patents, (III) construction of two claim terms, and (IV) decision to enhance damages. On cross-appeal, Sunoco challenged the district court’s decision not to grant lost-profits damages and its reasonable-royalty award.
The discussion is focused on the topic of on-sale-bar. Venture’s on-sale-bar defense, if successful, would invalidate some claims in two of Sunoco’s patents. The “on-sale-bar” provides the principle that “no person is entitled to patent an ‘invention’ that has been ‘on sale’ more than one year before filing a patent application” (i.e., before the critical date). “On sale” defined by the courts should meet two criteria that the invention is both “the subject of a commercial offer for sale” and “ready for patenting.” Pfaff v. Wells Elec’s., Inc., 525 U.S. 55 (1998). In addition, the on-sale bar can be negated if the patent owner demonstrates that the sale qualifies as “primarily for purposes of experimentation.”
The inventor’s company (MCE) offered to sell and install a butane-blending system to Equilon on February 9, 2000, two days before the critical date. The agreement indicated that Equilon would not pay anything for the machine, but would agree to purchase at least 500,000 barrels of butane from MCE over the next 5 years. The district court found that the equipment was being given away since “the contract did not require Equilon to pay MCE anything in exchange for the system in the normal course of events.” The district court later determined that Sunoco’s systems were sold for experimental-use purposes rather than commercial purposes. However, the Federal Circuit disagreed and opined that the sale “agreement bears ‘all the hallmarks of a commercial contract for sale.’”
The Federal Circuit states that “the agreement begins by expressly describing the transaction as a sale, without reference to any experimental purpose” as recited in the agreement:
MCE agrees to sell to Equilon, and Equilon agrees to purchase, the Equipment (as hereinafter defined) along with a license to use certain technology and software owned by MCE pertaining to the computerized blending of Butane and gasoline stocks, in consideration for the purchase and sale of Butane as set forth herein.
The section of the agreement characterized the sale’s commercial purpose and indicated that “MCE already ‘developed’ the relevant technology and equipment, that Equilon wanted to purchase it, and that MCE was willing to sell it, install it, and supply butane for it.”
Sunoco argued that a primarily experimental purpose is a section of the agreement entitled “Equipment Testing” including two sets of testing: pre-installation testing and post-installation testing. Sunoco drew an analogy to the Supreme Court’s seminal City of Elizabeth case. However, the Federal Circuit pointed out that “the nature of a street pavement,” the invention in the case of the City of Elizabeth, “is such that it cannot be experimented upon satisfactorily except on a highway, which is always public,” City of Elizabeth, 97 U.S. at 134. The Federal Circuit found that the testing was not done by Equilon but a third party. Sunoco also acknowledged that the test could have been done at any time prior to entering the deal with Equilon in January of 2000.
For the posit-installation testing, the section of the agreement states:
Upon completion of installation of the Equipment, MCE shall provide Equilon with written notice of such completion. Within three (3) days of said notice, Equilon shall (i) make all necessary arrangements within the Terminal to enable MCE to test the Equipment to determine whether the Equipment is properly blending butane, and (ii) provide notification to MCE that said arrangements have been made. MCE shall test the Equipment according
to parameters set forth in Schedule 1.10. MCE shall proceed with testing in a timely manner and have a period not to exceed ninety (90) days from the date of said notification by Equilon to complete its testing.
The Federal Circuit found that the post-installation tests were also not experiments, but are acceptance tests to confirm that the equipment “is properly blending butane”—that is, that it is working as promised. It further noted that there was no objective evidence that supported a conclusion that the “primary purpose” of the sale was “to conduct experimentation.”
The Federal Circuit further noted that the district court should have considered “whether the invention was under development, subject to testing, or otherwise still in its experimental stage at the time of the asserted sale.” But that consideration is not the question of Pfaff prong 1. The district court’s “still under development” observation would be better considered at Pfaff prong 2.
Therefore, the Federal Circuit reversed the district court’s experimental-use determination and remand the district court to assess whether the invention was “ready for patenting.”.
Takeaway
- Transactions without primary experimental-use purpose do not insulate from the on-sale bar.
Primer in Patent Enforcement
| May 26, 2022
NICHIA CORPORATION, v. DOCUMENT SECURITY SYSTEMS, INC.
Decided: April 26, 2022
Before DYK, REYNA, and STOLL. Opinion by REYNA
Summary
Although not precedential, this decision serves as a primer on patent enforcement and the interplay of district court actions by a patent owner, and defenses raised by an alleged infringer with a petition for inter partes review. A review of the underlying Board decision reveals eight district court cases brought by Document Security Systems, Inc., against six different entities, where three inter partes reviews were requested. There were also petitions for inter partes reviews (IPRs) of different patents owned by Document Security Systems, Inc. not involved in this appeal.
In one of the IPRs, the Board found claims 1, 6-8, 15 and 17 of USP 7,524,087 (the ‘087 patent which is the patent involved in the decision) unpatentable (Seoul Semiconductor Co. v. Document Sec. Sys., Inc., IPR2018-00522 and Everlight Elecs. Co. v. Document Sec. Sys., Inc., IPR 2018-01226 – motion for joinder with IPR 2018-00522). In another IPR, the Board declined to institute inter partes review due to the petition being filed more than one year after service of the complaint alleging infringement (Cree, Inc., v. Document Sec. Sys., Inc., IPR2018-01221).
In the present decision, the Board found claims 1 and 6-8 of the ‘087 patent unpatentable and found that claims 2-5 and 9-19 of the ‘087 patent are not unpatentable. Nichia appeals the decision upholding claims 2-5 and 9-19 while Document Security Systems appeals the holding that claims 1 and 6-8 are unpatentable. The CAFC affirmed the Board’s findings as to all claims except claims 15-19, reversing on claim 15 and remanding on dependent claims 16-19.
Background
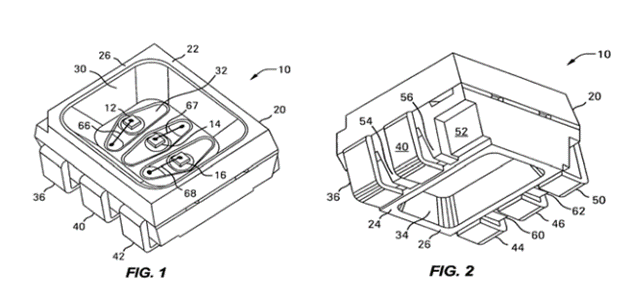
The ‘087 patent relates to an optical device with an LED die, such as for use in a large display panel. As an example, a stadium display may include numerous small light emitting elements arranged in an array and consisting of an LED die mounted to a plastic housing. In an exemplary embodiment, LEDs are mounted in a housing and encapsulated for protection from the environment. The device contains reflector housing 20 with a sidewall 26 extending between top 22 and bottom 24, with a first pocket or cavity 30 formed on top and a second pocket 34 formed on bottom shown in Figs. 1 and 2:
The first pocket 30 may be filled with encapsulant to cover and protect the LED dies. Claim 1 is representative.
1. An optical device comprising:
a lead frame with a plurality of leads;
a reflector housing formed around the lead frame, the reflector housing having a first end face and a second end face and a peripheral sidewall extending between the first end face and the second end face, the reflector housing having a first pocket with a pocket opening in the first end face and a second pocket with a pocket opening in the second end face;
at least one LED die mounted in the first pocket of the reflector housing;
a light transmitting encapsulant disposed in the first pocket and encapsulating the at least one LED die; and
wherein a plurality of lead receiving compartments are formed in the peripheral sidewall of the reflector housing.
The Board found that claims 1 and 6-8 are unpatentable as obvious over Takenaka (teaching most of claim 1) in combination with Kyowa (teaching the remaining limitation requiring multiple lead-receiving compartments in the reflector housing sidewall). The Board further found that Nichia did not demonstrate that claims 1 and 6-14 are unpatentable over Okazaki and Kyowa because Okazaki discloses a tubular vessel instead of the claimed two pockets. The Board found that Document Security’s expert was more credible than Nichia’s expert in that one of ordinary skill would understand Okazaki as teaching a tubular vessel instead of two pockets. The Board also found that Nichia did not demonstrate that claims 9-19 are unpatentable over Takenaka in view of Kyowa because Nichia did not identify disclosure in Takenaka regarding claim 9’s plastic limitation: “A display comprising a plurality of plastic leaded chip carrier LEDs, the plastic leaded chip carrier LEDs each comprising ….” The Board determined that the underlined phrase was not part of the preamble. Lastly, the Board found that Nichia also did not identify any disclosure in Takenaka that teaches or suggests the “electrical connection limitation” in independent claim 15.
Discussion
The CAFC first notes the standard of review: the Board’s legal conclusions are reviewed de novo whereas the factual findings underlying obviousness are reviewed for substantial evidence.
Nichia’s Appeal
Nichia makes three arguments that the Board erred in finding that Nichia failed to show that claims 9-19 are unpatentable over Takenaka in view of Kyowa.
In the first argument, Nichia argued that the Board erred in the construction of the preamble of claim 9. Nichia argued that the preamble should be considered as the entirety of “A display comprising a plurality of plastic leaded chip carrier LEDs, the plastic leaded chip carrier LEDs each” comprising” (the second “comprising” being considered the transitional phrase). The Board found the preamble to be “A display.” Claim construction is an issue of law reviewed de novo. The CAFC simply found that the phrase “[a] display comprising” is a general description followed by the transition word “comprising” and then the required elements.
In the second argument, Nichia argued that the Board abused its discretion by not including information contained in claim charts regarding plastic from Takenaka. The CAFC found that Nichia failed to establish anywhere in its petition or expert declaration that Takenaka disclosed “plastic.” Nichia’s claim charts disclosed use of resin with no argument comparing plastic and resin.
In the third argument, Nichia argued that the Board abused it’s discretion in finding that Nichia did not prove claim 15 unpatentable based on Takenaka in view of Kyowa. Claim 15 is similar to claim 1 but includes the additional limitation of “at least one LED die … electrically connected to said plurality of electrically conductive leads.” Nichia argues that the Board abused its discretion by ignoring Nichia’s reference to its claim chart for claim 1. Unlike with the plastic phrase, Nichia’s petition specifically stated that Takenaka disclosed an electrical connection. Therefore, the CAFC reversed the Board’s determination regarding claim 15 and remanded with respect to dependent claims 16-19.
Document Security’s Cross-Appeal
The cross-appeal challenged the determination that claims 1 and 6-8 are unpatentable over Takenaka in view of Kyowa. Document Security made two arguments. In the first argument, Document Security argued that Takenaka meets all the limitations of the asserted patent’s disclosed method to protect the leads from external forces, and therefore there was no need to combine with Kyowa.[1] Motivation to combine is a finding of fact. The Board relied on Nichia’s expert that a person of ordinary skill in the art would have been motivated to add the compartments described in Kyowa to the LED housing sidewall of Takenaka to protect the leads from external forces. The expert testimony and Kyowa’s disclosure provide “relevant evidence as a reasonable mind” would find supports the Board’s conclusion.
In the second argument, Document Security argued that even if there was motivation to combine, the Board erred because Kyowa does not teach a required element of the claims at issue, arguing that Kyowa does not disclose or suggest reflector housing and therefore cannot teach the lead-receiving compartments limitation. What the prior art teaches is a finding of fact. The Board explained that Kyowa teaches the device is enclosed in a resin package, which is an LED housing, and the sidewall contains multiple compartments in the housing. The Board reasoned that since Takenaka teaches a housing formed of white resin having high reflectance, it would correspond to the required reflector housing. As such, the CAFC found the Board’s findings supported by substantial evidence.
Takeaways
- Any IPR petition needs to clearly tie elements of the prior art to elements of the claim(s) at issue. For example, the resin of the prior art needed to be shown to correspond to plastic of claim 9. On the other hand, Nichia’s petition clearly set forth “electrical” connection which resulted in reversal.
[1] The author notes that this seems to be strange that the patent owner was arguing that it’s claim would be taught by one prior art reference. It is suspected that the strategy was to save the claim based on the multiple compartments limitation which was taught by the secondary reference.
Insufficient Extrinsic Evidence and Prosecution Disclaimer Led to Claim Construction in Patentee’s Favor
| May 6, 2022
Decided: April 1, 2022
Newman, Reyna, and Stoll. Opinion by Reyna.
Summary
The CAFC found in favor of a patentee seeking broad interpretation of a claim term, where an expert testimony offered by an opponent in support of narrower claim scope was insufficient to overcome a clear record of the patent, and the prosecution history lacked “clear and unmistakable” statements to establish express disavowal of claim scope.
Details
This is an appeal from a district court action where Genuine Enabling Technology LLC (“Genuine”) sued Nintendo Co., Ltd. and Nintendo of America, Inc. (“Nintendo”) for infringing certain claims of U.S. Patent No. 6,219,730. The patent relates to user input devices, such as mouse and keyboard, and its representative claim 1 recites:
1. A user input apparatus operatively coupled to a computer via a communication means additionally receiving at least one input signal, comprising:
user input means for producing a user input stream;
input means for producing the at least one input signal;
converting means for receiving the at least one input signal and producing therefrom an input stream; and
encoding means for synchronizing the user input stream with the input stream and encoding the same into a combined data stream transferable by the communication means.
(Emphasis added.)
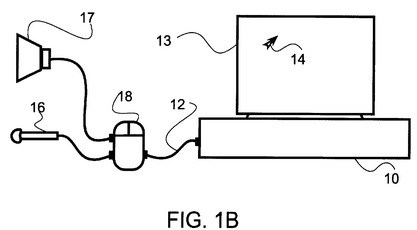
The inventive idea may be pictured as a “voice mouse.” As seen in Fig. 1B of the patent, the inventive apparatus or mouse 18, connected with a computer 10 via a communication link 12, serves the traditional function of allowing user to move an object 14 on the computer monitor 13 (i.e., “user input means for producing a user input stream”), while also capable of receiving a speech input from a microphone 16 (i.e., “input means for producing the at least one input signal”) and transmitting a speech output to a speaker 17.
At issue in the case is the construction of the term “input signal.”
During prosecution, the inventor distinguished his invention over U.S. Patent No. 5,990,866 (“Yollin”) cited in an office action, arguing that the “input signal” limitation was missing in the reference. Specifically, the inventor argued that various physiological sensors disclosed in Yollin, such as those detecting user’s muscle movement, heart rate, brain activity, blood pressure, skin temperature, etc., only produce “slow varying” signals as opposed to “signals containing audio or higher frequencies” to which his invention pertains. The inventor pointed out that the inventive apparatus would resolve a specific problem presented by the use of high frequency signals, which, unlike slow varying signals, tend to “collide” with other signals in producing a composite data stream. This “slow varying” vs. “audio or higher frequencies” distinction is repeatedly noted in the inventor’s argument.
The parties disputed the extent of prosecution disclaimer resulting from the inventor’s statements as to the term “input signal.” In the district court, Genuine proposed to construe the term as
“a signal having an audio or higher frequency,”
whereas Nintendo maintained that the proper scope should be narrower:
“A signal containing audio or higher frequencies. [The inventor] disclaimed signals that are 500 Hertz (Hz) or less. He also disclaimed signals that are generated from positional change information, user selection information, physiological response information, and other slow-varying information. Alternatively, indefinite.”
Nintendo argued that the inventor disclaimed the certain types of “slow-varying” signals taught by Yollin. While specific frequencies were not disclosed in Yollin, Nintendo relied on its expert testimony citing a reference purportedly showing that Yollin’s physiological sensors encompassed the range of “at least 500 Hz.”
Genuine contended that the disclaimed scope should be limited to “slow-varying signals below the audio frequency spectrum [of 20 Hz to 20,000 Hz],” in accordance with the inventor’s argument which only had distinguished the reference “slow-varying” signals from “fast-varying” signals.
The district court sided with Nintendo, finding non-infringement in its favor. In particular, the district court noted that “an applicant’s argument that a prior art reference is distinguishable on a particular ground can serve as a disclaimer of claim scope even if the applicant distinguishes the reference on other grounds as well.” Andersen Corp. v. Fiber Composites, LLC, 474 F.3d 1361, 1374 (Fed. Cir. 2007). That is, according to the district court, the fact that the inventor’s argument distinguished Yollin as lacking “audio or higher frequencies” did not negate the effect of the inventor’s statement also distinguishing Yollin’s range as “slow-varying” signals.
On appeal, the CAFC rejected the district court’s claim construction.
First, the CAFC noted that the role of extrinsic evidence in claim construction is limited, and cannot override intrinsic evidence where there is clear written record of the patent. And as one form of extrinsic evidence, “expert testimony may not be used to diverge significantly from the intrinsic record.” Here, the 500 Hz frequency threshold was not identified in the intrinsic record nor Yollin, and its only basis was Nintendo’s expert testimony which relied on another extrinsic reference that was not in the record. The extrinsic evidence conflicted with the prosecution history in which the examiner had accepted the inventor’s argument distinguishing Yollin without drawing a bright line between “slow-varying” and “audio or higher frequenc[y]” signals. As such, the CAFC found that the expert testimony cannot overcome the clarity of the intrinsic evidence.
Second, the CAFC noted that prosecution disclaimer requires that the patentee make a “clear and unmistakable” statement to disavow claim scope during prosecution. The fact that the inventor repeatedly distinguished the reference “slow-varying” signals from “audio or higher frequenc[y]” signals—the argument which successfully traversed the rejection—led the CAFC to decide that the “clear and unmistakable” disavowal was limited to “signals below the audio frequency spectrum.” Although the CAFC noted that the inventor’s statements “may implicate” certain frequency ranges such as 500 Hz or less, the intrinsic record was not unambiguous enough for the particular range to be disclaimed. For similar reasons, the CAFC also found no disclaimer of “signals that are generated from positional change information, user selection information, physiological response information, and other slow-varying information,” which had not been directly addressed in the inventor’s argument.
The CAFC concluded that the district court erred in the claim construction and that the proper scope of the term “input signal” is “a signal having an audio or higher frequency.”
Takeaway
This case provides a reminder that caution should be taken not to make “clear and unmistakable” statements in arguing around the prior art during prosecution. Although there appears to be no fixed criteria for assessing what qualifies as a “clear and unmistakable” disavowal, applicant’s consistency in presenting one main argument—focused on an essential distinction between the claim and the prior art—without introducing unnecessary details may help limit the extent of potential prosecution disclaimer.
Claim differentiation helps broaden the scope of a claim to a Fuse end cap
| April 29, 2022
Littlefuse, Inc. v. Mersen USA EP Corp.
Decided: April 4, 2022
Prost, Bryson and Stoll. Opinion by Bryson.
Summary:
Littlefuse sued Mersen for infringement of its patent to a fuse end cap. In construing the claims, the district court held that the claims are limited to a multi-piece apparatus even though some dependent claims recite a single-piece apparatus. Upon review of the claims, specification, and prosecution history, and based on principles of claim differentiation, the CAFC concluded that the district court’s construction is incorrect. The CAFC concluded that the independent claims should be construed as including multi-piece fuse end caps as well as single-piece fuse end caps. Thus, the CAFC vacated the district court’s judgment and remanded.
Details:
Littlefuse owns U.S. Patent No. 9,564,281 to a “fuse end cap for providing an electrical connection between a fuse and an electrical conductor.” Independent claim 1 is provided:
1. A fuse end cap comprising:
a mounting cuff defining a first cavity that receives an end of a fuse body, the end of the fuse body being electrically insulating;
a terminal defining a second cavity that receives a conductor, wherein the terminal is crimped about the conductor to retain the conductor within the second cavity; and
a fastening stem that extends from the mounting cuff and into the second cavity of the terminal that receives the conductor.
Dependent claims 8 and 9 are also important in this case and are provided:
8. The fuse end cap of claim 1, wherein the mounting cuff and the terminal are machined from a single, contiguous piece of conductive material.
9. The fuse end cap of claim 1, wherein the mounting cuff and the terminal are stamped from a single, contiguous piece of conductive material.
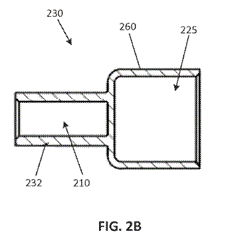
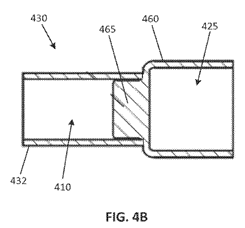
The specification describes three embodiments: (1) a “machined end cap” that may be manufactured from a single piece of any suitable, electrically conductive material; (2) a “stamped end cap” that may be manufactured from a single piece of any suitable, electrically conductive material; and (3) an “assembled end cap” in which the terminal and mounting cuff are formed from separate pieces of material, the material being any suitable, electrically conductive material. With the “assembled end cap,” the terminal and mounting cuff are “joined together, such as by press-fitting a fastening stem of the mounting cuff into the cavity of the terminal” or by using “a variety of other fastening means including … various adhesives, various mechanical fasteners, or welding.”
The following is an example of the “machined fuse end cap”:
And the following is an example of the “assembled end cap”:
During prosecution, the Examiner issued a restriction requirement stating that each of the three embodiments represented a distinct species. Littlefuse elected to prosecute the species corresponding to the “assembled end cap” embodiment. Claims 8 and 9 directed to the “machined end cap” and the “stamped end cap,” respectively, were then withdrawn. These dependent claims were subject to reinstatement if a generic claim was found to be allowable. Upon a rejection issued by the Examiner, claim 1 was amended to include the “fastening stem” limitation shown above. Amended claim 1 was then allowed and claims 8 and 9 were rejoined since the Examiner concluded that claims 8 and 9 require all the limitations of the allowable claims.
District Court
In the district court, the term “fastening stem” was construed to mean “a stem that attaches or joins other components”; and “a fastening stem that extends from the mounting cuff and into the second cavity of the terminal that receives the conductor” was construed to mean “a stem that extends from the mounting cuff and into the second cavity of the terminal that receives the conductor, and attaches the mounting cuff to the terminal.” The district court further construed claim 1 such that the fuse end cap “is of multi-piece construction” and that claim 1 does not cover a single-piece apparatus. The parties stipulated to non-infringement based on the district court’s construction of claim 1 as covering only a multi-piece apparatus, and Littlefuse appealed the claim construction ruling.
CAFC
The court first explained claim construction principles citing Phillips v. AWH Corp., 415 F.3d 1303 (Fed. Cir. 2005) (en banc) stating that “A claim term is generally given the meaning that the term would have to a person of ordinary skill in the art in question at the time of the invention” and that “How the term is used in the claims and the specification of the patent is strong evidence of how a person of ordinary skill would understand the term.”
The court further stated that “The structure of the claims is enlightening.” Citing Baxalta Inc. v. Genentech, Inc., 972 F.3d 1341 (Fed. Cir. 2020), the court stated that “By definition, an independent claim is broader than a claim that depends from it, so if a dependent claim reads on a particular embodiment of the claimed invention, the corresponding independent claim must cover that embodiment as well.” The court stated that if this were not the case, then the dependent claim would be meaningless, and a claim construction that leads to that result is generally disfavored. Thus, the court concluded that the recitation of a single-piece apparatus in claims 8 and 9 is persuasive evidence that claim 1 also covers a single-piece apparatus.
The court then explained principles regarding claim-differentiation stating that “the presumption of differentiation in claim scope is not a hard and fast rule” citing Seachange Int’l, Inc. v. C-COR, Inc., 413 F.3d 1361 (Fed. Cir. 2005) and that “any presumption created by the doctrine of claim differentiation will be overcome by a contrary construction dictated by the written description or prosecution history” citing Retractable Techs., Inc. v. Becton, Dickinson, and Co., 653 F.3d 1296 (Fed. Cir. 2011).
Mersen argued that notwithstanding the doctrine of claim differentiation, a construction that renders certain claims superfluous need not be rejected if that construction is consistent with the teachings of the specification citing Marine Polymer Technologies, Inc. v. HenCon, Inc., 672 F.3d 1350 (Fed. Cir. 2012) (en banc). However, the court stated that Littlefuse’s construction is supported by the specification. The court further stated that Mersen’s construction “would not merely render the dependent claims superfluous, but would mean that those claims would have no scope at all, a result that should be avoided when possible.”
The court pointed out that the district court recognized the inconsistency between its construction of claim 1 as covering only a multi-piece apparatus and the recitation of a single-piece apparatus in dependent claims 8 and 9. The district court concluded that the Examiner’s rejoinder of those dependent claims was a mistake. However, the CAFC said that the record in this case does not support the district court’s conclusion. The CAFC agreed with the Examiner that the dependent claims “require all the limitations of the … allowable claims” since the independent claims are not limited to a multi-piece construction and “the dependent claims form a coherent invention in that they each recite a fuse end cap that comprises a mounting cuff, a terminal, and a fastening stem.
The court also addressed the specification which only refers to a “fastening stem” with respect to the “assembled end cap” embodiment, which is a multi-piece apparatus. But the court said that “courts ordinarily should not limit the claimed invention to preferred embodiments or specific examples in the specification.” The court pointed out that the specification does not state that a fastening stem cannot be present in a single-piece apparatus and “one can envision a stem that projects from the side of the mounting cuff even in an embodiment in which the fuse end cap is formed from a single piece of material.”
The court concluded that the construction of “fastening stem” that is most consistent with the claims, specification, and prosecution history is a construction that includes a multi-piece end cap as well as a single-piece end cap. Thus, the court vacated the district court’s judgment of non-infringement and remanded.
Comments
Claim differentiation can be a useful tool for providing broader claim scope to independent claims. Thus, when drafting patent applications, it is a good idea to include various dependent claims.
Also, when faced with a restriction requirement due to distinct species and upon allowance of some claims, make sure to review whether other claims can be rejoined. In this case, the fact that the Examiner rejoined some species claims as being covered by a generic independent claim helped persuade the CAFC to provide a broader construction of the independent claim.
« Previous Page — Next Page »