Definitely Consisting Essentially Of: Basic and Novel Properties Must Be Definite Under 35 U.S.C. §112
| November 15, 2019
HZNP Medicines, LLC, Horizon Pharma USA, Inc. v. Actavis Laboratories UT, Inc.
October 15, 2019
Before: Prost, Reyna and Newman; Opinion by: Reyna; Dissent-in-part by: Newman
Summary: Horizon appealed a claim construction that the term “consisting essentially of” was indefinite. The district court evaluated the basic and novel properties under the Nautilus definiteness standard and found that the properties set forth in Horizon’s specification where indefinite. Horizon maintained this was legal error. The CAFC found that having used the phrase “consisting essentially of,” Horizon thereby incorporated unlisted ingredients or steps that do not materially affect the basic and novel properties of the invention. They asserted that a drafter cannot later escape the definiteness requirement by arguing that the basic and novel properties of the invention are in the specification, not the claims. Newman dissents, noting that no precedent has held that “consisting essentially of” composition claims are invalid unless they include the properties of the composition in the claims and that the majority’s ruling “sows conflict and confusion.”
Details:
- Background
HZNP Medicines, LLC, Horizon Pharma USA, Inc (“Horizon”) owns a series of patents directed to a methods and compositions for treating osteoarthritis. Actavis Laboratories UT, Inc (“Actavis”) sought to manufacture a generic version. Horizon sued Actavis for infringement in the District of New Jersey. Most of Horizon’s claims were removed by Summary Judgment on multiple issues. One claim survived to trial and was found valid and infringed. The CAFC taking up the appeal and cross-appeal on a number of issues (induced infringement, obviousness of a modified chemical formula and indefiniteness) affirmed the District Court.
This paper focuses on the ruling that Horizon claims using the “consisting essentially of” transitional phrase where invalid based on the basic and novel properties being indefinite under 35 U.S.C. §112.
- The CAFC’s Decision
Several of the claims in the Horizon patents recited a formulation “consisting essentially of” various ingredients. Claim 49 of the ’838 patent was used as the example.
49. A topical formulation consisting essentially of:
1–2% w/w diclofenac sodium;
40–50% w/w DMSO;
23–29% w/w ethanol;
10–12% w/w propylene glycol;
hydroxypropyl cellulose; and
water to make 100% w/w, wherein the topical formulation has a viscosity of 500–5000 centipoise.
The claims had been found invalid by the District Court under Summary Judgement following a Markman hearing. The parties’ dispute focused on the basic and novel properties of the claims. The CAFC agreed with the District Court that these properties are implicated by virtue of the phrase “consisting essentially of,” which allows unlisted ingredients to be added to the formulation so long as they do not materially affect the basic and novel properties.
The district court held that the specification of the patents identified five basic and novel properties: (1) better drying time; (2) higher viscosity; (3) increased transdermal flux; (4) greater pharmacokinetic absorption; and (5) favorable stability. Further, the district court reviewed the characteristics and found that at least “better drying time” was indefinite because the specifications provided two separate manners of determining drying time which were inconsistent. Based thereon, they concluded that the basic and novel properties of the claimed invention were indefinite under Nautilus.
The CAFC evaluated whether the Nautilus definiteness standard applies to the basic and novel properties of an invention. Horizon had argued that the Nautilus definiteness standard focuses on the claims and therefore does not apply to the basic and novel properties of the invention. The majority found this argument to be “misguided” and asserted that by using the phrase “consisting essentially of” in the claims, the inventor in this case incorporated into the scope of the claims an evaluation of the basic and novel properties.
The use of “consisting essentially of” implicates not only the items listed after the phrase, but also those steps (in a process claim) or ingredients (in a composition claim) that do not materially affect the basic and novel properties of the invention. Having used the phrase “consisting essentially of,” and thereby incorporated unlisted ingredients or steps that do not materially affect the basic and novel properties of the invention, a drafter cannot later escape the definiteness requirement by arguing that the basic and novel properties of the invention are in the specification, not the claims.
They supported this position by noting that a patentee can reap the benefit of claiming unnamed ingredients and steps by employing the phrase “consisting essentially of” so long as the basic and novel properties of the invention are definite.
In evaluating the district courts finding of indefiniteness, the CAFC maintained that two questions arise when claims use the phrase “consisting essentially of.” The first focusing on definiteness: “what are the basic and novel properties of the invention?” The second, focusing on infringement: “does a particular unlisted ingredient materially affect those basic and novel properties?” The definiteness inquiry focuses on whether a person of ordinary skill in the art (“POSITA”) is reasonably certain about the scope of the invention. If the POSITA cannot as-certain the bounds of the basic and novel properties of the invention, then there is no basis upon which to ground the analysis of whether an unlisted ingredient has a material effect on the basic and novel properties. Keeping with this logic, the CAFC maintained that to determine if an unlisted ingredient materially alters the basic and novel properties of an invention, the Nautilus definiteness standard requires that the basic and novel properties be known and definite.
Hence, the CAFC concluded that the district court did not err in considering the definiteness of the basic and novel properties during claim construction.
The majority then looked to the specific finding of indefiniteness by the district court regarding the basic and novel property of “better drying time.” They noted that the specification discloses results from two tests: an in vivo test and an in vitro test. The district court had found that the two different methods for evaluating “better drying time” do not provide consistent results at consistent times. Accordingly, CAFC affirmed the district court’s conclusion that the basic and novel property of “better drying rate” was indefinite, and consequently, that the term “consisting essentially of” was likewise indefinite.
In sum, we hold that the district court did not err in: (a) defining the basic and novel properties of the formulation patents; (b) applying the Nautilus definiteness standard to the basic and novel properties of the formulation patents; and (c) concluding that the phrase “consisting essentially of” was indefinite based on its finding that the basic and novel property of “better drying time” was indefinite on this record.
However, they noted that the Nautilus standard was not to be applied in an overly stringent manner.
To be clear, we do not hold today that so long as there is any ambiguity in the patent’s description of the basic and novel properties of its invention, no matter how marginal, the phrase “consisting essentially of” would be considered indefinite. Nor are we requiring that the patent owner draft claims to an untenable level of specificity. We conclude only that, on these particular facts, the district court did not err in determining that the phrase “consisting essentially of” was indefinite in light of the indefinite scope of the invention’s basic and novel property of a “better drying time.”
Judge Newman’s Dissent
Judge Newman dissented. She asserted that the majority holding that “By using the phrase ‘consisting essentially of’ in the claims, the inventor in this case incorporated into the scope of the claims an evaluation of the basic and novel properties” is not correct as a matter of claim construction, it is not the law of patenting novel compositions, and it is not the correct application of section 112(b).
First, she noted that there is no precedent that when the properties of a composition are described in the specification, the usage “consisting essentially of” the ingredients of the composition invalidates the claims when the properties are not repeated in the claims.
Second, in regard to the specifics of “drying time” for the case at hand, she noted that whatever the significance of drying time as an advantage of the claimed composition, recitation and measurement of this property in the specification does not convert the composition claims into invalidating indefiniteness because the ingredients are listed in the claims as “consisting essentially of.”
The role of the claims is to state the subject matter for which patent rights are sought. The usage “consisting essentially of” states the essential ingredients of the claimed composition. There are no fuzzy concepts, no ambiguous usages in the listed ingredients. There is no issue in this case of the effect of other ingredients…
Noting that there was no evidence that a POSITA would not understand the components of the composition claims with reasonable certainty, Newman concluded that since in the current case there are no other components asserted to be present, no “unnamed ingredients and steps” that even adopting the construction taken by the majority the claims are not subject to invalidity for indefiniteness.
Takeaways:
Added caution must be taken by patent prosecutors when electing to use the transitional phrase “consisting essentially of”. The specification needs to be clear as to what the basic and novel properties are and how they are determinable.
When using “consisting essentially of” incorporating the properties that are considered to be basic and novel into the claim language may help prevent ambiguity.
Tags: 35 U.S.C. §112 > basic and novel properties > consisting essentially of > definiteness
Presenting multiple arguments in prosecution risks prosecution history estoppel on each of them
| November 8, 2019
Amgen Inc. v. Coherus BioSciences Inc.
July 29, 2019
Before Reyna, Hughes, and Stoll. Opinion by Stoll.
Summary
The CAFC affirmed a district court decision holding that Amgen had failed to state a claim and dismissed Amgen’s suit against Coherus for patent infringement under the doctrine of equivalents, in view of Amgen’s clear and unmistakable disclaimer of claim scope during prosecution.
Details
Amgen Inc. and Amgen Manufacturing Ltd. (“Amgen”) sued Coherus BioSciences Inc. for infringement of U.S. Patent No. 8,273,707 (the ‘707 patent). The patent relates to methods of purifying proteins using hydrophobic interaction chromatography (“HIC”), in which a buffered salt solution containing the desired protein is poured into a HIC column and the proteins are bound to a column matrix while the impurities are washed out. However, only a limited amount of protein can bind to the matrix. If too much protein is loaded on the column, some of the protein will be lost to the solution phase before elution.
Conventionally, a higher salt concentration in a buffer solution is provided to increase the dynamic capacity of the HIC column, but the higher salt concentration causes protein instability. Amgen’s ‘707 patent discloses a process that increases the dynamic capacity of a HIC column by providing combinations of salts instead of using a single salt.
According to the ‘707 patent, any one of the three combinations of salts – citrate and sulfate, citrate and acetate, or sulfate and acetate – allows for a decreased concentration of at least one of the salts to achieve a greater dynamic capacity without compromising the quality of the protein separation.
During prosecution, the Examiner rejected the claims as being obvious in view of U.S. Patent No. 5,231,178 (“Holtz”). In reply to the Examiner’s rejection, Amgen argued that:
(1) The pending claims recite a particular combination of salts. No combinations of salts are taught nor suggested in Holtz;
(2) No particular combinations of salts recited in the pending claims are taught or suggested in Holtz; and
(3) Holtz does not teach dynamic capacity at all.
Amgen also attached a declaration from the inventor. The declaration stated that the use of the three salt combinations leads to substantial increases in the dynamic capacity of a HIC column and “[use] of this particular combination of salts greatly improves the cost-effectiveness of commercial manufacturing by reducing the number of cycles required for each harvest and reducing the processing time for each harvest.”
The Examiner again rejected Amgen’s argument and took the position that the prior art does disclose salts used in a method of purification and that adjustment of conditions was within the skill of an ordinary artisan. This time, in response to the Examiner’s position, Amgen replied that Holtz does not disclose any combination of salts and does not mention the dynamic capacity of a HIC column. In particular, Amgen stated that it was a “lengthy development path” when choosing a working salt combination and that merely adding a second salt would not have been expected to result in the invention. The Examiner allowed the claims.
In 2016, Coherus sought FDA approval to market a biosimilar version of Amgen’s pegfilgrastim product. In 2017, Amgen sued Coherus for infringing the ‘707 patent under the doctrine of equivalents because Coherus’s process did not match any of the three explicitly recited salt combinations in the ‘707 patent. Coherus moved to dismiss Amgen’s complaint for failure to state a claim.
The district court agreed to dismiss the complaint. The district court noted that during prosecution, Amgen had distinguished Holtz by repeatedly arguing that Holtz did not disclose “one of the particular, recited combinations of salts” in the two responses and in the declaration. The district court held that “[t]he prosecution history, namely, the patentee’s correspondence in response to two office actions and a final rejection, shows a clear and unmistakable surrender of claim scope by the patentee.” In addition, the district court held that “by disclosing but not claiming the salt combination used by Coherus, Amgen had dedicated that particular combination to the public.”
Amgen appealed.
The CAFC affirmed the district Court’s dismissal and found that the “prosecution history estoppel has barred Amgen from succeeding on its infringement claim under the doctrine of equivalents.”
In the appeal, Amgen argued that it only had distinguished Holtz by stating that Holtz does not disclose increasing any dynamic capacity or mention any salt combination. Amgen also argued that the prosecution history should not apply here because the last response filed prior to allowance did not make the argument that Holtz failed to disclose the particular salt combinations.
Regarding Amgen’s first point – that during prosecution, only dynamic capacity had been used to distinguish Holtz – the CAFC noted the three grounds on which Amgen has relied for distinguishing Holtz, and the fact that Amgen had quoted the declaration in support of the particular combination of salts recited in the claims. The CAFC explained that “separate arguments create separate estoppels as long as the prior art was not distinguished based on the combination of these various grounds.”
The CAFC also disagreed with Amgen’s second point (that prosecution history estoppel should not apply because of their last response), commenting that “there is no requirement that argument-based estoppel apply only to arguments made in the most recent submission before allowance… We see nothing in Amgen’s final submission that disavows the clear and unmistakable surrender of unclaimed salt combinations made in Amgen’s response.”
The CAFC held that the prosecution history estoppel applied and affirmed the District Court’s order dismissing Amgen’s complaint for failure to state a claim. The CAFC did not discuss whether Amgen dedicated unclaimed salt combinations to the public.
Takeaway
- Prosecution history estoppel can be triggered, not only by narrowing amendments, but also by arguments, even without any amendments.
- Presenting multiple arguments does not eliminate the risk of triggering estoppel for each of them.
- When writing an argument, avoid using expressions that are not recited in the claims (when discussing the claimed invention) or the cited documents (when discussing the state of the art).
The CAFC’s Holding that Claims are Directed to a Natural Law of Vibration and, thus, Ineligible Highlights the Shaky Nature of 35 U.S.C. 101 Evaluations
| November 1, 2019
American Axle & Manufacturing, Inc. v. Neapco Holdings, LLC
October 9, 2019
Opinion by: Dyke, Moore and Taranto (October 3, 2019).
Dissent by: Moore.
Summary:
American Axle & Manufacturing, Inc. (AAM) sued Neapco Holdings, LLC (Neapco) for alleged infringement of U.S. Patent 7,774,911 for a method of manufacturing driveline propeller shafts for automotive vehicles. On appeal, the Federal Circuit upheld the District Court of Delaware’s holding of invalidity under 35 U.S.C. 101. The Federal Circuit explained that the claims of the patent were directed to the desired “result” to be achieved and not to the “means” for achieving the desired result, and, thus, held that the claims failed to recite a practical way of applying underlying natural law (e.g., Hooke’s law related to vibration and damping), but were instead drafted in a results-oriented manner that improperly amounted to encompassing the natural law.
Details:
- Background
The case relates to U.S. Patent 7,774,911 of American Axle & Manufacturing, Inc. (AAM) which relates to a method for manufacturing driveline propeller shafts (“propshafts”) with liners that are designed to attenuate vibrations transmitted through a shaft assembly. Propshafts are employed in automotive vehicles to transmit rotary power in a driveline. During use, propshafts are subject to excitation or vibration sources that can cause them to vibrate in three modes: bending mode, torsion mode, and shell mode.
The CAFC focused on independent claims 1 and 22 as being “representative” claims, noting that AAM “did not argue before the district court that the dependent claims change the outcome of the eligibility analysis.”
| Claim 1 | Claim 22 |
| 1. A method for manufacturing a shaft assembly of a driveline system, the driveline system further including a first driveline component and a second driveline component, the shaft assembly being adapted to transmit torque between the first driveline component and the second driveline component, the method comprising: providing a hollow shaft member; tuning at least one liner to attenuate at least two types of vibration transmitted through the shaft member; and positioning the at least one liner within the shaft member such that the at least one liner is configured to damp shell mode vibrations in the shaft member by an amount that is greater than or equal to about 2%, and the at least one liner is also configured to damp bending mode vibrations in the shaft member, the at least one liner being tuned to within about ±20% of a bending mode natural frequency of the shaft assembly as installed in the driveline system. | 22. A method for manufacturing a shaft assembly of a driveline system, the driveline system further including a first driveline component and a second driveline component, the shaft assembly being adapted to transmit torque between the first driveline component and the second driveline component, the method comprising: providing a hollow shaft member; tuning a mass and a stiffness of at least one liner, and inserting the at least one liner into the shaft member; wherein the at least one liner is a tuned resistive absorber for attenuating shell mode vibrations and wherein the at least one liner is a tuned reactive absorber for attenuating bending mode vibrations. |
As explained by the CAFC, “[i]t was known in the prior art to alter the mass and stiffness of liners to alter their frequencies to produce dampening,” and “[a]ccording to the ’911 patent’s specification, prior art liners, weights, and dampers that were designed to individually attenuate each of the three propshaft vibration modes — bending, shell, and torsion — already existed.” The court further explained that in the ‘911 patent “these prior art damping methods were assertedly not suitable for attenuating two vibration modes simultaneously,” i.e., “shell mode vibration [and] bending mode vibration,” but “[n]either the claims nor the specification [of the ‘911 patent] describes how to achieve such tuning.”
The District Court concluded that the claims were directed to laws of nature: Hooke’s law and friction damping. And, the District Court held that the claims were ineligible under 35 U.S.C. 101. AAM appealed.
- The CAFC’s Decision
Under 35 U.S.C. 101, “any new and useful process, machine, manufacture, or composition of matter, or any new and useful improvement thereof” may be eligible to obtain a patent, with the exception long recognized by the Supreme Court that “laws of nature, natural phenomena, and abstract ideas are not patentable.”
Under the Supreme Court’s Mayo and Alice test, a 101 analysis follows a two-step process. First, the court asks whether the claims are directed to a law of nature, natural phenomenon, or abstract idea. Second, if the claims are so directed, the court asks whether the claims embody some “inventive concept” – i.e., “whether the claims contain an element or combination of elements that is sufficient to ensure that the patent in practice amounts to significantly more than a patent upon the ineligible concept itself.”
At step-one, the CAFC explained that to determine what the claims are directed to, the court focuses on the “claimed advance.” In that regard, the CAFC noted that the ‘911 patent discloses a method of manufacturing a driveline propshaft containing a liner designed such that its frequencies attenuate two modes of vibration simultaneously. The CAFC also noted that AAM “agrees that the selection of frequencies for the liners to damp the vibrations of the propshaft at least in part involves an application of Hooke’s law, which is a natural law that mathematically relates mass and/or stiffness of an object to the frequency that it vibrates. However, the CAFC also noted that “[a]t the same time, the patent claims do not describe a specific method for applying Hooke’s law in this context.”
The CAFC also noted that “even the patent specification recites only a nonexclusive list of variables that can be altered to change the frequencies,” but the CAFC emphasized that “the claims do not instruct how the variables would need to be changed to produce the multiple frequencies required to achieve a dual-damping result, or to tune a liner to dampen bending mode vibrations.”
The CAFC explained that “the claims general instruction to tune a liner amounts to no more than a directive to use one’s knowledge of Hooke’s law, and possibly other natural laws, to engage in an ad hoc trial-and-error process … until a desired result is achieved.”
The CAFC explained that the “distinction between results and means is fundamental to the step 1 eligibility analysis, including law-of-nature cases.” The court emphasized that “claims failed to recite a practical way of applying an underlying idea and instead were drafted in such a results-oriented way that they amounted to encompassing the [natural law] no matter how implemented.
At step-two, the CAFC stated that “nothing in the claims qualifies as an ‘inventive concept’ to transform the claims into patent eligible matter.” The CAFC explained that “this direction to engage in a conventional, unbounded trial-and-error process does not make a patent eligible invention, even if the desired result … would be new and unconventional.” As the claims “describe a desired result but do not instruct how the liner is tuned to accomplish that result,” the CAFC affirmed that the claims are not eligible under step two.
NOTE: In response to Judge Moore’s dissent, the CAFC explained that the dissent “suggests that the failure of the claims to designate how to achieve the desired result is exclusively an issue of enablement.” However, the CAFC expressed that “section 101 serves a different function than enablement” and asserted that “to shift the patent-eligibility inquiry entirely to later statutory sections risks creating greater legal uncertainty, while assuming that those sections can do work that they are not equipped to do.”
- Judge Moore’s Dissent
Judge Moore strongly dissented against the majority’s opinion. Judge Moore argued, for example, that:
- “The majority’s decision expands § 101 well beyond its statutory gate-keeping function and the role of this appellate court well beyond its authority.”
- “The majority’s concern with the claims at issue has nothing to do with a natural law and its preemption and everything to do with concern that the claims are not enabled. Respectfully, there is a clear and explicit statutory section for enablement, § 112. We cannot convert § 101 into a panacea for every concern we have over an invention’s patentability.”
- “Section 101 is monstrous enough, it cannot be that now you need not even identify the precise natural law which the claims are purportedly directed to.” “The majority holds that they are directed to some unarticulated number of possible natural laws apparently smushed together and thus ineligible under § 101.”
- “The majority’s validity goulash is troubling and inconsistent with the patent statute and precedent. The majority worries about result-oriented claiming; I am worried about result-oriented judicial action.”
Takeaways:
- When drafting claims, be mindful to avoid drafting a “result-oriented” claim that merely recites a desired “result” of a natural law or natural phenomenon without including specific steps or details setting establishing “how” the results are achieved.
- When drafting claims, be mindful that although under 35 U.S.C. 112 supportive details satisfying enablement are only required to be within the written specification, under 35 U.S.C. 101 supportive details satisfying eligibility must be within the claims themselves.
What’s a tool? What is functionality? Network monitoring claim held patent-eligible in split opinion
| October 29, 2019
SRI International, Inc., v. Cisco Systems, Inc.
March 20, 2019
Before Lourie, O’Malley, and Stoll. Opinion by Stoll. Dissenting opinion by Lourie.
Summary
The CAFC affirmed a district court decision holding that claims related to network security monitoring are patent-eligible. In the 2-1 opinion, the CAFC held that all of the asserted claims are patent eligible under §101 as not “directed to” an abstract idea under the first step of the Alice test, because the claims focus on an improvement in the functionality of computers and computer network technology.
The CAFC also affirmed the district court’s construction of the claim term “network traffic data,” summary judgment of no anticipation, and award of ongoing royalties but willful infringement and attorneys’ fees issues were vacated and remanded.
This presentation only addresses the issue of patent eligibility.
Details
SRI International, Inc. (“SRI”) sued Cisco Systems, Inc. (“Cisco”) for infringement of U.S. Patent Nos. 6,711,615 (‘615 patent) and 6,484,203 (‘203 patent). The ‘615 patent is a continuation of the ‘203 patent. The patents relate to network security by using network monitors to analyze the data on the network and generating and integrating reports of suspicious activity.
SRI proposed claim 1 of the ‘615 patent as representative claim while Cisco proposed claim 1 of the ‘203 patent. The CAFC noted that the claims are substantially similar, the difference in the list of categories of data not being material to any issue on appeal, and adopted claim 1 of ‘615 patent as the representative claim.
Claim 1 of the ‘615 patent:
1. A computer-automated method of hierarchical event monitoring and analysis within an enterprise network comprising:
deploying a plurality of network monitors in the enterprise network;
detecting, by the network monitors, suspicious network activity based on analysis of network traffic data selected from one or more of the following categories: {network packet data transfer commands, network packet data transfer errors, network packet data volume, network connection requests, network connection denials, error codes included in a network packet, network connection acknowledgements, and network packets indicative of well-known network-service protocols};
generating, by the monitors, reports of said suspicious activity; and
automatically receiving and integrating the reports of suspicious activity, by one or more hierarchical monitors.
As a preliminary matter, the CAFC noted that SRI spent considerable investment on network intrusion detection and developed the Event Monitoring Enabling Response or Anomalous Live Disturbances (“EMERALD”) project prior to the filing of the patents. In addition, the CAFC also noted that the Defense Advanced Research Projects Agency of the Department of Defense, which helped fund the project, called it a “gem in the world of cyber defense” and “‘a quantum leap improvement over’ previous technology.”
As to the issue of patent eligibility, the District Court held that the claims do more than merely recite the performance of a known business practice on the Internet and are better understood as being necessarily rooted in computer technology in order to solve a specific problem in the realm of computer networks. The CAFC agreed.
The CAFC explained that by the recitation of detecting an activity, receiving and integrating the reports, the claim does more than just the normal, expected operation of a conventional computer network. The CAFC specifically described the technological improvement as “a network defense system that monitors network traffic in real-time to automatically detect large-scale attacks,” with reference to Enfish, LLC v. Microsoft Corp.
In addition, the CAFC noted that the specification provided an explanation of both the technological problem – the network becomes more valuable when the technology become more interoperable and integrated but also makes it more vulnerable to attack – and the technological solution, by providing “a framework for the recognition of more global threats to interdomain connectivity, including coordinated attempts to infiltrate or destroy connectivity across an entire network enterprise.” Unfortunately, here, the CAFC simply cited the specification of the SRI patent but did not provide any details regarding how the specification presents the technological solution in relation to the claim language.
When arguing that the claims are directed to an abstract idea, Cisco had raised three main arguments, which the CAFC addressed in turn:
1. the claims are “directed to generic steps required to collect and analyze data,” therefore, “the claims are analogous to those in Electric Power Group, LLC v. Alstom S.A.” in which the claims were simply using the computers as tools. Thus, “the claims are directed to an abstract idea”;
2. “the invention does not involve an improvement to computer functionality itself”; and
3. the claims correspond generally to what people can “go through in their minds”.
Regarding Cisco’s first argument, the CAFC disagreed with Cisco’s view that the SRI patent claims are similar to the claims in Electric Power Group,[i] because the claimed invention in Electric Power Group was only “using computers as tools to solve a power grid problem.” The CAFC emphasized that the claims are similar to the claims in DDR Holdings, LLC v. Hotels.com, which were “directed to more than merely requiring a computer network operating in is normal, expected manner.”
Next, in rejecting Cisco’s second argument, the CAFC asserted that the representative claim is not about “automating a conventional idea on a computer” but “improv[ing] the technical functioning of the computer and computer networks by reciting a specific technique for improving computer network security.”
The CAFC also rejected Cisco’s third argument that the claims recite a mental process, by countering that “the human mind is not equipped to detect suspicious activity by using network monitors and analyzing network packets.”
In conclusion, the CAFC held that the claims at issue are not “directed to” an abstract idea under step one of Alice, because the claims are not just using the computer as a tool to analyze data from multiple sources to detect suspicious activity. Instead, the claims define using network monitors to detect suspicious network activity based on analysis of network traffic data, generating reports of that suspicious activity, and integrating those reports using hierarchical monitors to identify hackers and potential intruders. Thus, the claims provide an improvement in the functionality of computers and computer networks, and, therefore, the claims are patent eligible.
Dissent
Dissenting Judge Lourie thought that the claims of the SRI patent were similar to the claims in Electric Power Group because, in his view, the SRI claims “recite nothing more than deploying network monitors, detecting suspicious network activity, and generating and handling reports.” Judge Lourie noted that in Electric Power Group, the claims that were held patent-ineligible recited “receiving data,” “detecting and analyzing events in real time,” “displaying the event analysis results and diagnoses of events,” “accumulating and updating measurements,” and “deriving a composite indicator of reliability.”
Further, he pointed out that the portions of the SRI specification to which the majority refers “only recites results, not means for accomplishing them,” and that the SRI claims as written “do not recite a specific way of enabling a computer to monitor network activity.” Since he considered that the SRI claims do not provide any specifics as to how the steps are performed and show no improvement to computer technology, he would have held that the claims were directed to an abstract idea.
Takeaway
As this decision shows, there is much uncertainty regarding whether a claim would be treated as patent-eligible or not under the Alice test. It seems that a different set of judges might have easily sided with Judge Lourie’s analysis and invalidated the claims.
In the
meantime, the explanations and details given in the patent description
regarding the technical problem solved by the invention, instead of referring
to generic computer or network components, helped these claims survive the
patent-eligibility challenge.
[i] Claim 12 of U.S. Patent 8,401,701 at issue in Electric Power Group, LLC v. Alstom S.A.:
12. A method of detecting events on an interconnected electric power grid in real time over a wide area and automatically analyzing the events on the interconnected electric power grid, the method comprising:
receiving a plurality of data streams, each of the data streams comprising sub-second, time stamped synchronized phasor measurements wherein the measurements in each stream are collected in real time at geographically distinct points over the wide area of the interconnected electric power grid, the wide area comprising at least two elements from among control areas, transmission companies, utilities, regional reliability coordinators, and reliability jurisdictions;
receiving data from other power system data sources, the other power system data sources comprising at least one of transmission maps, power plant locations, EMS/SCADA systems;
receiving data from a plurality of non-grid data sources;
detecting and analyzing events in real-time from the plurality of data streams from the wide area based on at least one of limits, sensitivities and rates of change for one or more measurements from the data streams and dynamic stability metrics derived from analysis of the measurements from the data streams including at least one of frequency instability, voltages, power flows, phase angles, damping, and oscillation modes, derived from the phasor measurements and the other power system data sources in which the metrics are indicative of events, grid stress, and/or grid instability, over the wide area;
displaying the event analysis results and diagnoses of events and associated ones of the metrics from different categories of data and the derived metrics in visuals, tables, charts, or combinations thereof, the data comprising at least one of monitoring data, tracking data, historical data, prediction data, and summary data;
displaying concurrent visualization of measurements from the data streams and the dynamic stability metrics directed to the wide area of the interconnected electric power grid;
accumulating and updating the measurements from the data streams and the dynamic stability metrics, grid data, and non-grid data in real time as to wide area and local area portions of the interconnected electric power grid; and
deriving a composite indicator of reliability that is an indicator of power grid vulnerability and is derived from a combination of one or more real time measurements or computations of measurements from the data streams and the dynamic stability metrics covering the wide area as well as non-power grid data received from the non-grid data source.
[1] Claim 12 of U.S. Patent 8,401,701 at issue in Electric Power Group, LLC v. Alstom S.A.:
12. A method of detecting events on an interconnected electric power grid in real time over a wide area and automatically analyzing the events on the interconnected electric power grid, the method comprising:
receiving a plurality of data streams, each of the data streams comprising sub-second, time stamped synchronized phasor measurements wherein the measurements in each stream are collected in real time at geographically distinct points over the wide area of the interconnected electric power grid, the wide area comprising at least two elements from among control areas, transmission companies, utilities, regional reliability coordinators, and reliability jurisdictions;
receiving data from other power system data sources, the other power system data sources comprising at least one of transmission maps, power plant locations, EMS/SCADA systems;
receiving data from a plurality of non-grid data sources;
detecting and analyzing events in real-time from the plurality of data streams from the wide area based on at least one of limits, sensitivities and rates of change for one or more measurements from the data streams and dynamic stability metrics derived from analysis of the measurements from the data streams including at least one of frequency instability, voltages, power flows, phase angles, damping, and oscillation modes, derived from the phasor measurements and the other power system data sources in which the metrics are indicative of events, grid stress, and/or grid instability, over the wide area;
displaying the event analysis results and diagnoses of events and associated ones of the metrics from different categories of data and the derived metrics in visuals, tables, charts, or combinations thereof, the data comprising at least one of monitoring data, tracking data, historical data, prediction data, and summary data;
displaying concurrent visualization of measurements from the data streams and the dynamic stability metrics directed to the wide area of the interconnected electric power grid;
accumulating and updating the measurements from the data streams and the dynamic stability metrics, grid data, and non-grid data in real time as to wide area and local area portions of the interconnected electric power grid; and
deriving a composite indicator of reliability that is an indicator of power grid vulnerability and is derived from a combination of one or more real time measurements or computations of measurements from the data streams and the dynamic stability metrics covering the wide area as well as non-power grid data received from the non-grid data source.
The limitations of a “wherein” clause
| October 15, 2019
Allergan Sales, LLC v. Sandoz, Inc., No. 2018-2207
August 29, 2019
Prost, Newman and Wallach. Opinion by Wallach
Summary
Appellees (Allergan hereon in) sued Appellants (Sandoz hereon in) asserting that their Abbreviated New Drug Application (ANDA) for a generic version of Allergan’s ophthalmic drug (Combigan®) infringed on their U.S. Patent Nos. 9,770,453: 9,907,801: 9,907,802. The District Court found limiting a number of “wherein” clauses in the Patents’ and granted Allergan’s motion for Preliminary Injection. Sandoz appealed. CAFC affirmed.
As an exemplary claim, independent claim 1 of the ‘453 patent is as follows:
A method of treating a patient with glaucoma or ocular hypertension comprising topically administering twice daily to an affected eye a single composition comprising 0.2% w/v brimonidine tartrate and 0.68% w/v timolol maleate,
wherein the method is as effective as the administration of 0.2% w/v brimonidine tartrate monotherapy three times per day and
wherein the method reduces the incidence of one o[r] more adverse events selected from the group consisting of conjunctival hyperemia, oral dryness, eye pruritus, allergic conjunctivitis, foreign body sensation, conjunctival folliculosis, and somnolence when compared to the administration of 0.2% w/v brimonidine tartrate monotherapy three times daily.
The specifications contained a clinical study, referred to as Example II, and it is the results thereof that are reflected in “the disputed “wherein” clauses (i.e., the efficacy and safety of the claimed combination).
Allergan argued that the “wherein” clauses were limiting, whereas Sandoz argued that the “wherein” clauses were not. Specifically, Sandoz argued that the “wherein” clauses “merely state the intended results” and so are not “material to patentability.” Sandoz argued that the only positive limitation in the claim[s] is the administering step. Sandoz relied upon previous cases to argue that “…whereby [or wherein] clause in a method claim is not given weight when it simply expresses the intended result of a process step positively recited.”); Bristol–Myers Squibb Co. v. Ben Venue Labs., Inc., 246 F.3d 1368, 1376 (Fed. Cir. 2001).
The District Court and CAFC disagreed. The courts looked to the specification, and to the prosecution history wherein the Applicant had relied upon the results of administering the combination drug to assert patentability over the prior art. It was also noted that the Examiner had explicitly relied upon the “wherein” clauses in his explanation as to why the claims were novel and non-obvious over the prior art in the Notice of Allowance.
The courts differentiated this case from the previous case argued by Sandoz in that, “In Bristol–Myers we expressly noted that the disputed claim terms “w[ere] voluntarily made after the examiner had already indicated . . . the claims were allowable” and such “unsolicited assertions of patentability made during prosecution do not create a material claim limitation.”
Accordingly, “the District Court “f[ound] that the ‘wherein’ clauses are limiting because they are material to patentability and express the inventive aspect of the claimed invention” and the CAFC affirmed.
Take-away
“The specification is always highly relevant to the claim construction analysis and is, in fact, the single best guide to the meaning of a disputed term.” Prosecution history and the Examiner’s express rational for allowance are also highly relevant.
WHEN FIGURES IN A DESIGN PATENT DO NOT CLEARLY SHOW AN ARTICLE OF MANUFACTURE FOR THE ORNAMENTAL DESIGN, THE TITLE AND CLAIM LANGUAGE CAN LIMIT THE SCOPE OF THE DESIGN PATENT
| October 7, 2019
Curver Luxembourg, SARL, v. Home Expressions Inc.
September 12, 2019
Chen (author), Hughes, and Stoll
Summary:
The Federal Circuit affirmed the district court’s grant of a defendant’s motion to dismiss a complaint for failure to state a plausible claim of design patent infringement because when all of the drawings in a design patent at issue do not describe an article of manufacture for the ornamental design, the title, claim language, figure descriptions specifying an article of manufacture, which was amended during the prosecution of the patent based on the Examiner’s proposed amendment, can limit the scope of a design patent.
Details:
The ‘946 Patent
Curver Luxembourg, SARL (Curver) is the assignee of U.S. Design Patent No. D677,946 (‘946 patent) with a title “Pattern for a Chair” and claiming an “ornamental design for a pattern for a chair.” The ‘946 patent claims an overlapping “Y” design, as shown below. However, none of the figures illustrate a design being applied to a chair.
Prosecution
Curver originally applied for a patent directed to a pattern for “furniture,” and the original title was “FURNITURE (PART OF-).” The original claim recited a “design for a furniture part.”
However, during the prosecution, the Examiner allowed the claim but objected to the title because it was too vague to constitute an article of manufacture (in Ex Parte Quayle Action). The Examiner suggested amending the title to read “Pattern for a Chair,” and Curver accepted the Examiner’s suggestion by replacing the title with “Pattern for a Chair” and amending the claim term “furniture part” with “pattern for a chair.” Curver did not amend the figures to illustrate a chair. The Examiner accepted Curver’s amendments and allowed the application.


District Court
Home Expressions makes and sells baskets with a similar overlapping “Y” design disclosed in the ‘946 patent.
Curver sued Home Expressions accusing its basket products of infringing the ‘946 patent. Home Expressions filed a motion to discuss Curver’s complaint under Rule 12(b)(6) for failing to set forth a plausible claim of infringement.

Using a two-step analysis, the district court construed the scope of the ‘946 patent to be limited to the design pattern illustrated in the figures as applied to a chair and found that an ordinary observer would not purchase Home Expressions’s basket with “Y” design believing that the purchase was for “Y” design applied to a chair.
Therefore, the district court granted the Rule 12(b)(6) motion.
CAFC
The CAFC held that to define the scope of a design patent, the court traditionally focused on the figures illustrated in the patent. However, when all of the drawings fail to describe an article of manufacture for the ornamental design, the CAFC held that claim language specifying an article of manufacture can limit the scope of a design patent.
In addition, the CAFC uses §1.153(a) to held that “the design be tied to a particular article, but this regulation permits claim language, not just illustration along, to identify that article.”
The title of the design must designate the particular article. No description, other than a reference to the drawing, is ordinarily required. The claim shall be in formal terms to the ornamental design for the article (specifying name) as shown, or as shown and described.
The CAFC held that the prosecution history shows that Curver amended the title, claim, figure descriptions to recite “pattern for a chair” in order to satisfy the article of manufacture requirement necessary to secure its design patent. Therefore, the CAFC held that the scope of the ‘946 patent should be limited by those amendments.
Therefore, the CAFC affirmed the district court’s grant of Home Expressions’s motion to dismiss the complaint for failure to state a plausible claim of design patent infringement.
Takeaway:
- When figures in a design patent do not clearly show an article of manufacture for the ornamental design, the title and claim language can limit the scope of the design patent.
- Applicant should review the Examiner’s proposed amendments carefully before placing the application in condition for allowance.
- Applicant should be careful when crafting the title and claim language.
Tags: article of manufacture > claim > Design Patent > figure > patent infringement > title
A Good Fry: Patent on Oil Quality Sensing Technology for Deep Fryers Survives Inter Partes Review
| October 4, 2019
Henny Penny Corporation v. Frymaster LLC
September 12, 2019
Before Lourie, Chen, and Stoll (Opinion by Lourie)
Summary
In an appeal from an inter partes review, the Federal Circuit affirmed the Patent Trial and Appeal Board’s decision to uphold the validity of a patent relating to oil quality sensing technology for deep fryers. The Board found, and the Federal Circuit agreed, that the disadvantages of pursuing the challenger’s proposed modification of the prior art weighed against obviousness, in the absence of some articulated rationale as to why a person of ordinary skill in the art would have pursued that modification. In addition, the Federal Circuit reiterated that as a matter of procedure, the scope of an inter partes review is limited to the theories of unpatentability presented in the original petition.
Details
Fries are among the most common deep-fried foods, and McDonald’s fries may still be the most popular and highest-consumed worldwide. But, there would be no McDonald’s fries without a deep fryer and a good pot of oil, and Frymaster LLC (“Frymaster”) is the maker of some of McDonald’s deep fryers.
During deep frying, chemical and thermal interactions between the hot frying oil and the submerged food cause the food to cook. These interactions degrade the quality of the oil. In particular, chemical reactions during frying generate new compounds, including total polar materials (TPM), that can change the oil’s physical properties and electrical conductivity.
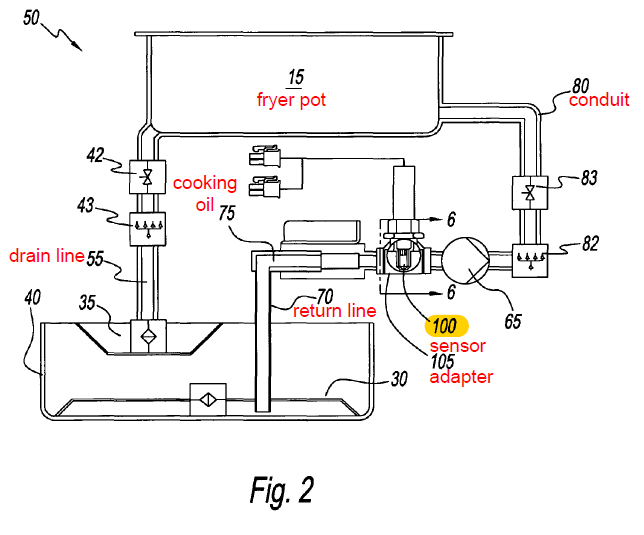
Frymaster’s fryers are equipped with integrated oil quality sensors (OQS), which monitor oil quality by measuring the oil’s electrical conductivity as an indicator of the TPM levels in the oil. This sensor technology is embodied in Frymaster’s U.S. Patent No. 8,497,691 (“691 patent”).
The 691 patent describes an oil quality sensor that is integrated directly into the circulation of cooking oil in a deep fryer, and is capable of taking measurements at the deep fryer’s operational temperatures of 150-180°C, i.e., without cooling the hot oil.
Claim 1 of the 691 patent is representative:
1. A system for measuring the state of degradation of cooking oils or fats in a deep fryer comprising:
at least one fryer pot;
a conduit fluidly connected to said at least one fryer pot for transporting cooking oil from said at least one fryer pot and returning the cooking oil back to said at least one fryer pot;
a means for re-circulating said cooking oil to and from said fryer pot; and
a sensor external to said at least on[e] fryer pot and disposed in fluid communication with said conduit to measure an electrical property that is indicative of total polar materials of said cooking oil as the cooking oil flows past said sensor and is returned to said at least one fryer pot;
wherein said conduit comprises a drain pipe that transports oil from said at least one fryer pot and a return pipe that returns oil to said at least one fryer pot,
wherein said return pipe or said drain pipe comprises two portions and said sensor is disposed in an adapter installed between said two portions, and
wherein said adapter has two opposite ends wherein one of said two ends is connected to one of said two portions and the other of said two ends is connected to the other of said two portions.
Figure 2 of the 691 patent illustrates the structure of Frymaster’s system:
Henny Penny Corporation (HPC) is a competitor of Frymaster, and initiated an inter partes review of the 691 patent.
In its petition, HPC challenged claim 1 of the 691 patent as being obvious over Kauffman (U.S. Patent No. 5,071,527) in view of Iwaguchi (JP2005-55198).
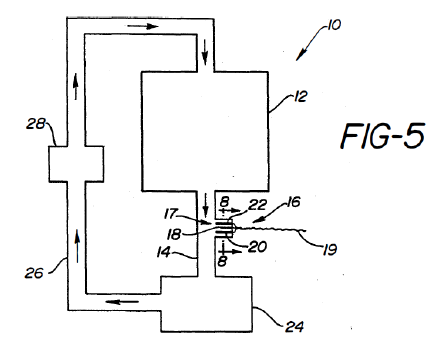
Kauffman taught a system for “complete analysis of used oils, lubricants, and fluids”. The system included an integrated electrode positioned between drain and return lines connected to a fluid reservoir. The electrode measured conductivity and current to monitor antioxidant depletion, oxidation initiator buildup, product buildup, and/or liquid contamination. Kauffman’s system operated at 20-400°C. However, Kauffman did not teach monitoring TPMs.
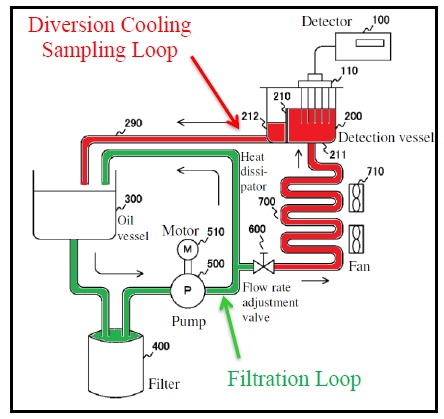
Iwaguchi taught monitoring TPMs to gauge quality of oil in deep fryers. However, Iwaguchi cooled the oil to 40-80°C before taking measurements. If the oil temperature was outside the disclosed range, Iwaguchi’s system would register an error. Specifically, oil was diverted from the frying pot to a heat dissipator where the oil was cooled to the appropriate temperature, and then to a detection vessel where a TPM detector measured the electrical properties of the oil to detect TPMs. Iwaguchi taught that cooling relieved heat stress on the detector, prevented degradation, and obviated the need for large conversion tables.
The parties’ dispute focused on the sensor feature of the 691 patent.
In the initial petition, HPC argued simply that a person of ordinary skill in the art would have found it obvious to modify Kauffman’s system to “include the processor and/or sensor as taught by Iwaguchi.”
In its patent owner’s response, Frymaster disputed HPC’s proposed modification. Frymaster argued that Iwaguchi’s “temperature sensitive” detector would be inoperable in an “integrated” system such as that taught in Kauffman, unless Kauffman’s system was further modified to add an oil diversion and cooling loop. However, such an addition would have been complex, inefficient, and undesirable to those skilled in the art.
In its reply, HPC changed course and argued that it was unnecessary to swap the electrode in Kauffman’s system for Iwaguchi’s detector. HPC argued that Kauffman’s electrode was capable of monitoring TPMs by measuring conductivity, and that Iwaguchi was relevant only for teaching the general desirability of using TPMs to assess oil quality.
However, whereas HPC’s theory of obviousness in its original petition was based on a modification of the physical structure of Kauffman’s system, HPC’s reply proposed changing only the oil quality parameter being measured. During the oral hearing before the Board, HPC’s counsel even admitted to this shift in HPC’s theory of obviousness.
In its final written decision, the Board determined, as a threshold matter of procedure, that HPC impermissibly presented a new theory of obviousness in its reply, and that the patentability of the 691 patent would be assessed only against the grounds asserted in HPC’s original petition.
The Board’s final written decision thus addressed only whether the person skilled in the art would have been motivated to “include”—that is, integrate—Iwaguchi’s detector into Kauffman’s system. The Board found no such motivation.
The Board’s reasoning largely mirrored Frymaster’s arguments. Kauffman’s system did not include a cooling mechanism that would have allowed Iwaguchi’s temperature-sensitive detector to work. Integrating Iwaguchi’s detector into Kauffman’s system would therefore necessitate the addition of the cooling mechanism. The Board agreed that the disadvantages of such additional construction outweighed the “uncertain benefits” of TPM measurements over the other indicia of oil quality already being monitored in Kauffman.
On appeal, HPC raised two issues: first, the Board construed the scope of HPC’s original petition overly narrowly; and second, the Board erred in its conclusion of nonobviousness.[1]
The Federal Circuit sided with the Board on both issues.
On the first issue, the Federal Circuit did a straightforward comparison of HPC’s petition and reply. In the petition, HPC proposed a physical substitution of Iwaguchi’s detector for Kauffman’s electrode. In the reply, HPC proposed using conductivity measured by Kauffman’s electrode as a basis for calculating TPMs. The apparent differences between the two theories of obviousness, together with the “telling” confirmation of HPC’s counsel during oral hearing that the original petition espoused a physical modification, made it easy for the Federal Circuit to agree with the Board’s decision to disregard HPC’s alternative theory raised in its reply.
The Federal Circuit reiterated the importance of a complete petition:
It is of the utmost importance that petitioners in the IPR proceedings adhere to the requirement that the initial petition identify ‘with particularity’ the ‘evidence that supports the grounds for the challenge to each claim.
On the second issue of obviousness, HPC argued that the Board placed undue weight on the disadvantages of incorporating Iwaguchi’s TPM detector into Kauffman’s system.
Here, the Federal Circuit reiterated “the longstanding principle that the prior art must be considered for all its teachings, not selectively.” While “[t]he fact that the motivating benefit comes at the expense of another benefit…should not nullify its use as a basis to modify the disclosure of one reference with the teachings of another”, “the benefits, both lost and gained, should be weighed against one another.”
The Federal Circuit adopted the Board’s findings on the undesirability of HPC’s proposed modification of Kauffman, agreeing that the “tradeoffs [would] yield an unappetizing combination, especially because Kauffman already teaches a sensor that measures other indicia of oil quality.”
At first glance, the nonobviousness analysis in this decision seems to involve weighing the disadvantages and advantages of the proposed modification. However, looking at the history of this case, I think the problem with HPC’s arguments was more fundamentally that they never identified a satisfactory motivation to make the proposed modification. HPC’s original petition argued that the motivation for integrating Iwaguchi’s detector into Kauffman’s system was to “accurately” determine oil quality. This “accuracy” argument failed because it was questionable whether Iwaguchi’s detector would even work at the operating temperature of a deep fryer. Further, HPC did not argue that the proposed modification was a simple substitution of one known sensor for another with the predictable result of measuring TPMs. And when Kauffman argued that the substitution was far from simple, HPC failed to counter with adequate reasons why the person skilled in the art would have pursued the complex modification.
Takeaway
- The petition for a post grant review defines the scope of the proceeding. Avoid being overly generic in a petition for post grant review. It may not be possible to fill in the details later.
- Context matters. If an Examiner is selectively citing to isolated disclosures in a prior art out of context, consider whether the context of the prior art would lead away from the claimed invention.
- The MPEP is clear that the disadvantages of a proposed combination of the prior art do not necessarily negate the motivation to combine (see, e.g., MPEP 2143(V)). The disadvantages should preferably nullify the Examiner’s reasons for the modification.
[1] On the issue of obviousness, HPC also objected to the Board’s analysis of Frymaster’s proffered evidence of industry praise as secondary considerations. This objection is not addressed here.
A Change in Language from IPR Petition to Written Decision May Not Result in A Change in Theory of Motivation to Combine
| September 25, 2019
Arthrex, Inc. v. Smith & Nephew, Corp.
August 21, 2019
Dyk, Chen, Opinion written by Stoll
Summary
The CAFC affirmed the Board’s decision that claims 10 and 11 of Patent ‘541 were obvious over Gordon in view of West and that the IPR proceeding was constitutional. The CAFC held that minor variations in wording, from the IPR Petition to the written Final Decision, do not violate the Administrative Procedure Act (APA). Further, the CAFC rejected Arthrex’s argument to reconsider evidence that was contrary to the Board’s decision since there was sufficient evidence to support the Board’s findings of obviousness by the preponderance of evidence standard.
Details
Arthrex’s Patent No. 8,821,541 (hereinafter “Patent ’541”) is directed towards a surgical suture anchor that reattaches soft tissue to bone. A feature of the suture anchor of Patent ‘541 is that the “fully threaded suture anchor’ includes ‘an eyelet shield that is molded into the distal part of the biodegradable suture anchor’”, wherein the eyelet shield is an integrated rigid support that strengthen the suture to the soft tissue. Id. at 2 and 3. The specification discloses that “because the support is molded into the anchor structure (as opposed to being a separate component), it ‘provides greater security to prevent pull-out of the suture.’ Id at col 5 ll. 52-56.” Id. at 3. Figure 5 of Patent ‘541 illustrates an embodiment of the suture anchor (component 1), wherein component 9 is the eyelet shield (integral rigid support) and component 3 is the body wherein helical threading is formed.
Claims 10 and 11 of Patent ‘541 are herein enclosed (emphasis added to the claim terms at issue in the dispute)
10. A suture anchor assembly comprising:
an anchor body including a longitudinal axis, a proximal end, a distal end, and a central passage extending along the longitudinal axis from an opening at the proximal end of the anchor body through a portion of a length of the anchor body, wherein the opening is a first suture opening, the anchor body including a second suture opening disposed distal of the first suture opening, and a third suture opening disposed distal of the second suture opening, wherein a helical thread defines a perimeter at least around the proximal end of the anchor body;
a rigid support extending across the central passage, the rigid support having a first portion and a second portion spaced from the first portion, the first portion branching from a first wall portion of the anchor body and the second portion branching from a second wall portion of the anchor body, wherein the third suture opening is disposed distal of the rigid support;
at least one suture strand having a suture length threaded into the central passage, supported by the rigid support, and threaded past the proximal end of the anchor body, wherein at least a portion of the at least one suture strand is disposed in the central passage between the rigid support and the opening at the proximal end, and the at least one suture strand is disposed in the first suture opening, the second suture opening, and the third suture opening; and
a driver including a shaft having a shaft length, wherein the shaft engages the anchor body, and the suture length of the at least one suture strand is greater than the shaft length of the shaft.
11. A suture anchor assembly comprising:
an anchor body including a distal end, a proximal end having an opening, a central longitudinal axis, a first wall portion, a second wall portion spaced opposite to the first wall portion, and a suture passage beginning at the proximal end of the anchor body, wherein the suture passage extends about the central longitudinal axis, and the suture passage extends from the opening located at the proximal end of the anchor body and at least partially along a length of the anchor body, wherein the opening is a first suture opening that is encircled by a perimeter of the anchor body, a second suture opening extends through a portion of the anchor body, and a third suture opening extends through the anchor body, wherein the third suture opening is disposed distal of the second suture opening;
a rigid support integral with the anchor body to define a single-piece component, wherein the rigid support extends across the suture passage and has a first portion and a second portion spaced from the first portion, the first portion branching from the first wall portion of the anchor body and the second portion branching from the second wall portion of the anchor body, and the rigid support is spaced axially away from the opening at the proximal end along the central longitudinal axis; and
at least one suture strand threaded into the suture passage, supported by the rigid support, and having ends that extend past the proximal end of the anchor body, and the at least one suture strand is disposed in the first suture opening, the second suture opening, and the third suture opening.
Smith & Nephew (hereinafter “Smith”) initiated an inter partes review of claims 10 and 11 of Patent ‘541. Smith alleged that claims 10 and 11 of Patent ‘541 were invalid as obvious over Gordon (U.S. Patent Application Publication No. 2006/0271060) in view of West (U.S. Patent No. 7,322,978) and alleged that claim 11 was anticipated by Curtis (U.S. Patent No. 5,464,427) (The discussion regarding Curtis (U.S. Patent No. 5,464,427) is not herein included). Smith argued that Gordon disclosed all the features claimed except for the rigid support. Gordon disclosed “a bone anchor in which a suture loops about a pulley 182 positioned within the anchor body. Figure 23 illustrates the pully 182 held in place in holes 184a, b.” Id. at 5 and 6.
Figure 23 of Gordon
Smith acknowledged that the pulley of Gordon was not “integral with the anchor body to define a single-piece component”, a feature recited in claim 11 of Patent ‘541. Smith cited West to allege obviousness of this feature. West also disclosed a bone anchor wherein one or more pins are fixed within the bore of the anchor body. In West, “to manufacture the bone anchor, ‘anchor body 12 and posts 23 can be cast and formed in a die. Alternatively anchor body 12 can be cast or formed and post 23a and 23b inserted later.’” Id. at 7.
Figure 1 of West
Smith argued that it would have been obvious to a skilled artisan to form the bone anchor of Gordon by the casting process of West to thereby create a rigid support integral with the anchor body to define a single piece component, as recited in claim 11 of Patent ‘541. Smith’s expert testified that the casting process of West would “minimize the materials used in the anchor, thus facilitating regulatory approval, and would reduce the likelihood of the pulley separating from the anchor body.” Id. at 7. Smith asserted that the casting process of West was well-known in the art and that such a manufacturing process “would have been a simple design choice.” Id. at 7. Arthrex argued that a skilled artisan would not have been motivated to modify Gordon in view of West. The Board agreed with Smith that the claims were unpatentable. Arthrex filed an appeal.
Arthrex appealed the Board’s decision that Smith proved unpatentability of claims 10 and 11 in view of Gordon and West, on both a procedurally and a substantively basis, and the constitutionality of the IPR proceeding. The CAFC found that Arthrex’s procedural rights were not violated; that the Decision by the Board was supported by substantial evidence and the application of IPR to Patent ‘541 was constitutional.
IPR proceedings are formal administrative adjudications subject to the procedural requirements of the APA. See, e.g., Dell Inc. v. Acceleron, LLC, 818 F.3d 1293, 1298 (Fed. Cir. 2016); Belden, 805 F.3d at 1080. One of these requirements is that “‘an agency may not change theories in midstream without giving respondents reasonable notice of the change’ and ‘the opportunity to present argument under the new theory.’” Belden, 805 F.3d at 1080 (quoting Rodale Press, Inc. v. FTC, 407 F.2d 1252, 1256–57 (D.C. Cir. 1968)); see also 5 U.S.C. § 554(b)(3). Nor may the Board craft new grounds of unpatentability not advanced by the petitioner. See In re NuVasive, Inc., 841 F.3d 966, 971–72 (Fed. Cir. 2016); In re Magnum Oil Tools Int’l, Ltd., 829 F.3d 1364, 1381 (Fed. Cir. 2016).
Id. at 9.
Arthrex argued that the Board’s decision relied on a new theory of motivation to combine. Arthrex argued that the new theory violated their procedural rights by depriving them an opportunity to respond to said new theory. Arthrex argued that the Board’s reasoning differed from the Smith Petition by describing the casting method of West as “preferred.” The CAFC held that while the language used by the Board did differ from the Petition, said deviation did not introduce a new issue or theory as to the reason for combining Gordon and West. West disclosed “an ‘anchor body 12 and posts 23 can be cast and formed in a die. Alternatively anchor body 12 can be cast or formed and posts 23a and 23b inserted later’.” Id. at 10. The Smith Petition asserted that “a person of ordinary skill would have had ‘several reasons’ to combine West and Gordon, including that the casting process disclosed by West was a ‘well-known technique [whose use] would have been a simple design choice’.” (emphasis added) Id. at 10. In the written decision by the Board, the Board characterized West as disclosing two manufacturing methods, wherein the casting method was the “primary” and “preferred” method, and thus, a skilled artisan would have “applied West’s casting method to Gordon because choosing the ‘preferred option’ presented by West ‘would have been an obvious choice of the designer’.” Id. at 11. The CAFC noted that while the Board’s language of “preferred” differed from the language of the Petition, i.e. “well-known”, “accepted” and “simple”, the Board nonetheless relied upon the same disclosure of West as the Petition, the same proposed combination of the references and ruled on the same theory of obviousness, as presented in the Petition. “[t]he mere fact that the Board did not use the exact language of the petition in the final written decision does not mean it changed theories in a manner inconsistent with the APA and our case law…. the Board had cited the same disclosure as the petition and the parties had disputed the meaning of that disclosure throughout the trial. Id. As a result, the petition provided the patent owner with notice and an opportunity to address the portions of the reference relied on by the Board, and we found no APA violation.” Id. at 11.
Next, Arthrex argued that even if the Board’s decision was procedurally proper, an error was made by finding Smith had established a motivation to combine the cited art by a preponderance of the evidence. The CAFC held that there was sufficient substantial evidence to support the Board’s finding that a skilled artisan would be motivated to use the casting method of West to form the anchor of Gordon. First, the Board noted West’s disclosure of two methods of making a rigid support. Second, the wording by West suggested that casting was the preferred method. Third, Smith’s experts testified that the casting method would likely produce a stronger anchor, which in turn would be more likely to be granted regulatory approval. Also, casting would decrease manufacturing cost, produce an anchor that is less likely to interfere with x-rays and would reduce stress concentrations on the anchor. The CAFC agreed with Arthrex that there was some evidence that contradicts the Board’s decision, such as Arthrex’s expert’s testimony. However, “the presence of evidence supporting the opposite outcome does not preclude substantial evidence from supporting the Board’s fact finding. See, e.g., Falkner v. Inglis, 448 F.3d 1357, 1364 (Fed. Cir. 2006)”. Id. at 14. The CAFC refused to “reweight the evidence.” Id. at 14.
Lastly, the CAFC addressed Arthrex’s argument that an IPR is unconstitutional when applied retroactively to a pre-AIA patent. Since this argument was first presented on appeal, the CAFC could have chosen not to address it. However, exercising its discretion, the CAFC held that an IPR proceeding against Patent ‘541 was constitutional. Patent ‘541 was filed prior to the passage of the AIA, but was issued almost three years after the passage of the AIA and almost two years after the first IPR proceedings, on September 2, 2014. “As the Supreme Court has explained, ‘the legal regime governing a particular patent ‘depend[s] on the law as it stood at the emanation of the patent, together with such changes as have since been made.’” Eldred v. Ashcroft, 537 U.S. 186, 203 (2003) (quoting McClurg v. Kingsland, 42 U.S. 202, 206 (1843)). Accordingly, application of IPR to Arthrex’s patent cannot be characterized as retroactive.” Id. at 18. The CAFC further explained that even if Patent ‘541 issued prior to the AIA, an IPR would have been constitutional. “[t]he difference between IPRs and the district court and Patent Office proceedings that existed prior to the AIA are not so significant as to ‘create a constitutional issue’ when IPR is applied to pre-AIA patents.” Id. at 18.
Takeaway
- Slight changes in language does not create a new theory of motivation to combine.
- However, a new theory of motivation to combine
may exist when:
- the Board
creates a new theory of obviousness by mixing arguments from two different
grounds of obviousness presented in a Petition.
- In re Magnum Oil Tools Int’l, Ltd., 829 F.3d 1364, 1372–73, 1377 (Fed. Cir. 2016).
- the claim construction by the Board varies
significantly from the uncontested construction announced in an institution
decision.
- SAS Institute v. ComplementSoft, LLC, 825 F.3d 1341, 1351 (Fed. Cir. 2016).
- the Board
relies upon a portion of the prior art, as an essential part of its obviousness
finding, that is different from the portions of the prior art cited in the
Petition.
- In re NuVasive, Inc., 841 F.3d 966, 971 (Fed. Cir. 2016).
- the Board
creates a new theory of obviousness by mixing arguments from two different
grounds of obviousness presented in a Petition.
Examples and procedures in the specification may provide necessary objective boundaries for a term of degree in the claims
| September 18, 2019
Guangdong Alison Hi-Tech Co., v. ITC, Aspen Aerogels, Inc. (intervenor)
August 27, 2019
Wallach, Hughes, and Stoll (Opinion author).
Summary
The ALJ at ITC held, later affirmed by the Commission, that the claims of U.S. Patent No. 7,078,359 were not invalid due to indefinites and anticipation and were infringed by Alison’s importation of the accused products. The Federal Circuit affirmed the validity determination.
Details
The ‘359 patent, titled “Aerogel Composite with Fibrous Batting,” is directed to an improvement in aerogel composite products. Specifically, the ‘359 patent specifically discloses an aerogel composite that uses a lofty batting to reinforce the aerogel in a way that maintains or improves the thermal properties of the aerogel while providing a highly flexible, drapeable form. The ‘359 patent is an improvement over prior aerogel composites, which suffer from low flexibility, low durability, and degraded thermal performance. Independent claim 1 of the ‘359 patent is:
1. A composite article to serve as a flexible, durable, light-weight insulation product, said article comprising a lofty fibrous batting sheet and a continuous aerogel through said batting.
Regarding the indefinites, the ALJ and the Commission rejected the Alison’ indefinite argument arguing that the phrase “lofty fibrous batting” in claim 1 is indefinite. The Federal circuit affirmed based on the “reasonable certainty” indefiniteness standard. Specifically, as set forth in Nautilus, Inc. v. Biosig Instruments, Inc., a patent’s claims are definite if the claims, viewed in light of the specification and prosecution history, inform those skilled in the art about the scope of the invention with reasonable certainty. A patentee need not define his invention with mathematical precision in order to comply with the definiteness requirement. Patents with claims involving terms of degree must provide objective boundaries for those of skill in the art in the context of the invention. Intrinsic evidence such as the claims, figures, written description, or prosecution history of a patent can provide the necessary objective boundaries. In this case, the Federal Circuit acknowledges that the phrase “lofty … batting” is a term of degree. However, the Federal Circuit held that the written description of the ‘359 patent provides objective boundaries for the term. The ‘359 patent specification first expressly defines “lofty … batting” as “a fibrous material that shows the properties of bulk and some resilience (with or without full bulk recovery).” The specification explains that “bulk” refers to the air or openness created by the web of fibers in a lofty batting. It further explains that a batting is “sufficiently resilient” if it “can be compressed to remove the air (bulk) yet spring back to substantially its original size and shape.” Furthermore, the specification details functional characteristics of a “lofty … batting.” Furthermore, the specification provides specific examples of commercial products that can qualify as a lofty batting, and a detailed discussion of seven examples of aerogel composites manufactured in accordance with the claimed invention, along with corresponding test results. Furthermore, during the prosecution, in the Statement of Reasons for allowance, the examiner distinguished the prior art based on the phrase “lofty fibrous batting.” Thus, the Federal Circuit categorize this case in the same class as previous cases like Sonix and Enzo, where examples and procedures in the written description provided sufficient guidance and points of comparison to render claim terms not indefinite.
The Federal Circuit also specifically rejected several Alison’ arguments in support of its indefiniteness challenge. First, Alison argued that the ‘359 patent provides no objective boundary between “some resilience,” which would infringe, and “little to no resilience,” which would not. In other words, in Alison’ view, the ‘359 patent fails to disclose precisely how much resilience is enough to satisfy the claim. The Federal Circuit dismissed this argument by holding that Alison was seeking a level of numerical precision beyond that required when using a term of degree. Next, Alison argued that the ‘359 patent offers two independent approaches, a thermal properties’ approach and a compressibility and resilience approach, to assess loftiness, without indicating which approach to use. The Federal Circuit dismissed this argument by holding that Alison has not provided any evidence that the different methods of measurement described in the ‘359 patent lead to different results. Lastly, Alison argued that the Commission’s indefiniteness analysis is irreconcilable contradiction with its claim construction. The ALJ provided a single reason for holding the claim term not indefinite because the specification states that “a lofty batting is sufficiently resilient if after compression for a few seconds it will return to at least 70% of its original thickness.” Yet, in construing the term, the ALJ declined to limit “lofty … batting” to this specific example of “resilience” in the specification, namely, “it will return to at least 70% of its original thickness.” The Federal Circuit dismissed this argument by holding that examples in the specification may be used to inform those skilled in the art of the scope of the invention with reasonable certainty, thus demonstrating that the term is not indefinite, without being directly construed into the claim.
Regarding the anticipation, Alison uses the same reference Ramamurthi, which was considered by the patent examiner during prosecution and later by the Board in denying Alison’s IPR petition. One of Alison’s argument is that Ramamurthi recites “glass wool” as a preferred fiber, which should be synonymous with the “fiber glass” expressly identified as a lofty batting by the ‘359 patent. However, the Commission rejected Alison’s anticipation argument based on Aspen’s detailed expert testimony, which demonstrated that “fiberglass” and “glass wool” each describe broad categories of materials that are not inherently “lofty.” the Federal Circuit held that the Commission’s determination is supported by substantial evidence, and rejected to reweight the evidence.
In conclusion, the Federal Circuit affirmed that the claims of the ‘359 patent are valid.
Take away
- In drafting specification, the drafter should provide some detailed examples of the invention, which can provide sufficient guidance and points of comparison to render certain claims terms definite.
- In defining claims terms in the specification, numerical precision is not necessarily needed to satisfy the definiteness requirement.
No obviousness where hindsight argument relied upon “cherry-picked” data
| September 11, 2019
Sanofi-Aventis et al. v. Dr. Reddy’s Laboratories et al.
August 14, 2019
Lourie, Moore, Taranto. Opinion by Lourie
Summary
The CAFC upheld the nonobviousness of claims where the lead compound was known, but no cited art would have motivated the skilled artisan to arrive at the claimed compound. The claimed compound was a specific modification of the lead compound, and no analogous modification was found in the art. The CAFC rejected “cherry-picked” data which formed a “convoluted” obviousness argument, and stated that there is no per se rule that a compound is obvious where there is only a small change from a lead compound.
Details
Technical background
Sanofi owns U.S. Patent Nos. 5,847,170 and 8,927,592, which claim cabazitaxel (commercially known as Jevtana) and methods of use thereof. This drug is used to treat drug-resistant prostate cancers. Cabazitaxel is only the third taxane drug to obtain FDA approval, since the first being in 1992. Cabazitaxel is similar to docetaxel (approved in 1996) as shown below:
Rather than hydroxyl groups at the C7 and C10 positions, Cabazitaxel has methoxy groups.
Procedural background
The Defendants filed an ANDA alleging that the ‘592 and ‘170 patents are invalid as being obvious. While the district court case arising out of the ANDA litigation was pending, the PTAB instituted an IPR of the ‘592 patent. The PTAB found some claims 1-5 and 7-30 of the ‘592 patent unpatentable as being obvious and denied Sanofi’s motion to amend. Sanofi appealed the denial of the motion to amend (this has been remanded to PTAB in another CAFC case from earlier in 2019), but did not appeal the invalidity decision with respect to claims 7, 11, 14-16 and 26. Sanofi filed a statutory disclaimer with respect to these claims.
Back at the district court, even though the statutory disclaimer had been filed and the district court was notified, the court held that a case or controversy still existed with respect to the disclaimed claims, and held that they were obvious. The court also held that claims 1 and 2 of the ‘170 patent were not invalid as being obvious. Sanofi appealed the district court’s conclusion that a case or controversy exists with respect to the claims of the ‘592 patent, and some of the defendants appealed the district court’s decision regarding the lack of obviousness of claims 1 and 2 of the ‘170 patent.[1]
District Court
The defendants argued that cabazitaxel would have been obvious in view of docetaxel as a lead compound. The district court based its conclusion of non-obviousness on seven witnesses and 17 prior art references. The court found that a person skilled in the art would have been motivated to use docetaxel as a lead compound, but would not have been motivated to replace the C7 and C10 hydroxyl groups with methoxy groups to arrive at cabazitaxel.
The defendants alleged that it would have been obvious to modify docetaxel by increasing lipophilicity, in order to interfere with a protein called Pgp, which is involved in drug resistance. The defendants cited to two references, Hait and Lampidis.
In Hait, the authors studied phenothiazines, not taxanes. They showed that by increasing lipophilicity, the cancer cell would be more sensitive to the drug. However, this related to a different class of drugs, which are structurally very different from taxanes. Also, Hait only disclosed hypothetical binding models with respect to Pgp.
In Lampidis, the authors found that increasing the lipophilicity of a positively charged dye caused accumulation in drug resistant cells. But, Lampidis also never disclosed taxanes. Further, taxanes do not have a positive charge, unlike the dye of Lampidis.
Next, the district court considered a reference (Commercon) that identified the C2’, C3’, C7, C9 and C10 positions of paclitaxel as “flexible” and suitable for modification, in combination several other references regarding the activity of taxane analogs. Cumulatively, the district court found that the defendants had “cherry-picked” data from the references. The district court was persuaded by Sanofi’s expert who explained that taxane modifications had been considered at positions C2, C4, C5, C7, C8, C9, C10, C11, C12, C13, C14, C2’ and C3’, but that it would not have been obvious to modify just C7 and C10 to be methoxy groups.
The district court also found secondary considerations. Despite significant efforts by others, cabazitaxel was only the third taxane to get FDA approval. Furthermore, Jevtana achieved significant commercial success.
CAFC
At the CAFC, the defendants argued that that the district court erred, and that one skilled in the art (1) would have been motivated to modify docetaxel to decrease Pgp-related drug resistance, (2) knew that this could be done by increasing lipophilicity at the C7 and C10 positions, and (3) knew that the methoxy substitutions were the most conservative modifications.
However, the CAFC agreed with Sanofi that this obviousness theory is based on hindsight and said that it is “convoluted.” First, the CAFC indicated that Hait and Lampidis would not have provided a reason to make docetaxel more lipophilic, for the same reasons as the district court. But, even if there was a reason to make docetaxel more lipophilic, the cited art was highly varied in its suggested modifications of the lead compound. No single reference taught any type of simultaneous change at the C7 and C10 positions, and certainly not methoxy substitutions.
As to the Commercon reference, the defendants argued that C7, C9 and C10 are taught to be flexible, but that C3’ and C2’ were “crucial.” However, the text of the reference itself stated that C3’ is flexible and C2’ can be modified under certain conditions.
Finally, the defendants argued that even though no reference shows only the C7 and C10 methoxy substitutions, this would have been obvious because they are known to be small, conservative changes that increase lipophilicity. They pointed to a methylthiomethoxy substitution as also suggesting a methoxy substitution. The CAFC refuted this as hindsight, since there was no evidence that these two types of substitutions would behave similarly.
The defendants also argued that where there are a small number of changes to try, it is obvious to make a single change to a lead compound, particularly where the change is known to have desirable properties. Again, there was no showing of beneficial properties from methoxy substitutions of C7 and C10 in a taxane. The CAFC also stated that small changes are not prima facie obvious.
Finally, the CAFC found no clear error with the secondary considerations and affirmed the district court analysis of non-obviousness of the claims.
Takeaway
-If an obviousness argument relies on data that can be fairly characterized as “cherry picked,” odds are it is an unsuccessful hindsight argument.
-There
is no per se rule that a “small change” from a lead compound results in a
conclusion of obviousness.
[1] This decision also includes an extensive discussion of why the district court did not have authority to hold that there is a case or controversy regarding disclaimed claims. Discussion of that issue is omitted here.
« Previous Page — Next Page »