CRITICALITY OF CLAIM CONSTRUCTION
| November 16, 2020
ST. JUDE MEDICAL, LLC, Appellant v. SNYDERS HEART VALVE LLC, Cross-Appellant UNITED STATES, Intervenor
October 15, 2020
Newman, O’Malley and Taranto (Opinion by Taranto)
This precedential decision illustrates the importance of claim construction, particularly in considering all terms of a proposed claim construction.
St. Jude filed two petitions for inter partes reviews of U.S Patent No. 6,540,782 owned by Snyders Heart Valve, LLC. The petitions were instituted as “IPR-105” and “IPR-106”. The ‘782 patent is directed to artificial heart valves. The key claim term in IPR-105 was a “band” whereas the key term in IPR-106 was a “manipulator.”
Standards of review are always important and worth noting. Anticipation is a question of fact in which the Board’s determination of what is taught in the prior art reviewed for substantial evidence. For obviousness, the ultimate determination is a legal one reviewed de novo, with underlying factual determinations reviewed for substantial evidence support. Claim construction under the broadest reasonable interpretation is a determination of law reviewed de novo where based on intrinsic evidence with any findings about facts based on extrinsic evidence reviewed for substantial evidence support.
IPR-105
The ‘782 patent describes an artificial heart valve that can be installed via catheter without invasive surgery. The artificial heart valve has three main components: a valve element, a frame, and a band. Independent claim 1 sets forth:
“a band attached to the frame limiting spacing between adjacent anchors of said plurality of peripheral anchors;”
IPR-105 was instituted on the basis that Leonhardt disclosed an artificial heart valve having three main components: a biological valve, a valve stent, and graft material. The graft material is made of low-porosity woven fabric which is arranged to surround the stent.
The Board had essentially adopted St. Jude’s proposed claim construction of the term “band” as “a structure generally in the shape of a closed strip or ring.” This construction was slightly broader than St. Jude’s originally proposed construction of “a structure generally in the shape of a circular strip or ring” to cover shapes including ovals for example. Nonetheless, the Board concluded that graft material of Leonhardt would not be considered a “band” under this claim construction since the graft material of Leonhardt covered the entire length of the stent like a sleeve, and therefore was not a “strip” or “ring.”
St. Jude argued that the Board had erred in not accepting that Leonhardt anticipated claims 1, 2, 4-8 and 28 on the basis that the graft material is a “band.” St. Jude argued that the Board failed to apply its own claim construction of “band” and instead applied a narrower construction. St. Jude argued that one of ordinary skill in the art would understand that there would be no width restriction on what is considered a “band” quoting a dictionary definition of “band” as a thin strip of flexible material used to encircle or bind one object or hold a number of objects.
The CAFC noted a fundamental problem with this argument in that it ignores the terms “strip” and “ring” which were accepted as part of the claim construction. If St. Jude wanted to argue that “band” should not have been limited to a “strip” or “ring’, it should have proposed a different claim construction.
IPR-106
In IPR-106, the Board concluded that St. Jude had proved that claims 1, 2, 6 and 8 were anticipated by Bessler, but that St. Jude failed to prove that Bessler disclosed a “manipulator” required by claim 28. Both St. Jude and Snyders challenged these conclusions.
Snyders challenged the finding of anticipation in regard to the Board’s claim construction of how the frame is “sized and shaped” as well as claim 1’s “attached” limitation. Snyders also challenged the finding that the cuff in Bessler satisfies the requirement of claim 1 that the band limits spacing between adjacent frame members. The CAFC agreed that the Board erred in construing the “sized and shaped” limitation and that Bessler would not anticipate.
Claim 1 requires a “frame sized and shaped for insertion between the upstream region and the downstream region.” The Board determined that “[t]he claim language does not require the frame be sized and shaped for insertion into a damaged heart valve,” but “only that the frame is sized and shaped for insertion in a position between the upstream region and the downstream region.” The Board did not dispute the express assertion by Snyders that Bessler requires removal of the native valve for installation of the replacement valve.
The CAFC pointed out that claim 1 requires “an artificial heart valve for repairing a damaged heart valve having a plurality of cusps, separating an upstream region from a downstream region … comprising …a flexibly resilient frame sized and shaped for insertion between the upstream region and downstream region” which provides support for Snyders’ assertion. The reference to “repairing” without removal suggests the native valve remains. The CAFC further highlighted the teachings of the specification for clarifying the broadest reasonable interpretation. The specification teaches:
“the frame is sized and shaped for insertion between the plurality of cusps C of the damaged heart valve in a position between an upstream region and a downstream region.”
This language indicates that “sized and shaped” is not meant to refer only to placement but also to fitting between cusps of the intact native heart. The CAFC concluded that the specification went beyond stating a general preference for leaving the native valve intact to make it unreasonable to read “sized and shaped for insertion” as covering an artificial valve fitted for space after removing the native valve.
St. Jude had relied on its claim construction that the claims cover the situation of a removed native valve and did not dispute the express assertion by Snyders that Bessler requires removal of the native heart valve. As such, the CAFC concluded that Bessler does not anticipate and therefore reverses the Board.
St. Jude challenged the finding that claim 28 was not anticipated in regard to the term “manipulator.” The CAFC concluded that it did not need to address this argument because claim 28 contains the “sized and shaped” limitation noted above.
St. Jude also challenged the finding that St. Jude failed to establish obviousness. Although the Board agreed that prior art advanced by St. Jude could be combined, the Board found that the particular combination proposed by St. Jude would not result. The Board found no adequate or persuasive explanation why one of ordinary skill in the art would keep the “integral” cuff portion while replacing the leaflet portion to which it is attached.
Takeaways
Claim construction is critical. Make sure to understand all terms of a proposed claim construction before accepting.
When challenging an obviousness determination based on a combination of art, remember that even if it would be reasonable to combine the art, the combination may not result in the particular arrangement as claimed.
The Patent Trial and Appeal Board has discretion in issuing sanctions and is not limited by the Sanctions Regulations under Rule 42.12
| November 5, 2020
APPLE INC. v. VOIP-PAL.COM, INC.
September 25, 2020
Prost, Reyna and Hughes. Opinion by Reyna.
Summary:
After being sued by Voip-Pal, Apple filed separate IPR petitions against the two patents asserted against Apple. During the IPRs, Voip-Pal sent letters to PTAB judges and the USPTO, among others, criticizing the IPR process and requesting either dismissal of the IPRs or favorable judgment. These letters were not sent to Apple. The PTAB issued a final written decision upholding the validity of the claims. Apple then filed a motion for sanctions due to the ex parte communications. The PTAB held that the letters sent by Voip-Pal were sanctionable and the remedy was to provide a new PTAB panel to review Apple’s request for rehearing. On appeal at the CAFC, but before the oral arguments, a separate CAFC decision issued affirming the invalidity of some of the same claims from the same patents. The CAFC stated that for the same claims involved in the prior case, the claims are invalid, and thus, the appeal is moot. However, for the claims that do not overlap with the claims involved in the prior case, the appeal is not moot. Regarding sanctions, the CAFC stated that the PTAB did not abuse its discretion in the remedy provided to Apple. The CAFC also affirmed the PTAB’s determination of non-obviousness.
Details:
Voip-Pal sued Apple for infringement of U.S. Patent Nos. 8,542,815 and 9,179,005 to Producing Routing Messages for Voice Over IP Communications. The patents describe routing communications between two different types of networks. Apple filed two separate IPR petitions for the patents. Apple agued that the patents were obvious over the combination of U.S. Patent No. 7,486,684 (Chu ‘684) and U.S. Patent No. 8,036,366 (Chu ‘366). The original PTAB panel instituted the IPRs. Later the original PTAB panel was replaced with a second PTAB panel. No reason for the change in panels was provided in the record.
While the IPRs were proceeding, the CEO of Voip-Pal sent six letters to various parties copying members of Congress, the President, federal judges, and administrative patent judges at the PTAB. The letters were not sent to Apple. The letters criticized the IPR system and requested judgment in Voip-Pal’s favor or dismissal of Apple’s IPRs.
The second PTAB panel then issued its final written decision for both IPRs. The claims were found to be not invalid as obvious over Chu ‘684 and Chu ‘366. Specifically, the PTAB said that Apple did not provide evidentiary support for their argument on motivation to combine.
Apple moved for sanctions against Voip-Pal due to the CEO’s ex parte communications with the PTAB and the USPTO arguing that the communications violated the Administrative Procedure Act and violated Apple’s due process rights. Apple requested an adverse judgment against Voip-Pal or that the final written decision be vacated and assigned to a new panel with a new proceeding. Apple also appealed the final written decision by the second PTAB panel.
The CAFC stayed the appeal and remanded to the PTAB to consider Apple’s sanctions motions. A third and final PTAB panel replaced the second PTAB panel for the sanctions proceedings. The final PTAB panel found that Voip-Pal’s ex parte communications were sanctionable. But the final PTAB panel rejected Apple’s request for a directed judgment or a new proceeding with a new panel. The final PTAB panel provided their own sanctions remedy of presiding over Apple’s request for rehearing. The final PTAB panel stated that this “achieves the most appropriate balance when considering both parties’ conduct as a whole.”
After briefing for the rehearing, the final PTAB panel denied Apple’s petition for rehearing because Apple had “not met its burden to show that in the Final Written Decision, the [Interim] panel misapprehended or overlooked any matter.” The final PTAB panel also stated that “even if [the final PTAB panel] were to accept [Apple’s] view of Chu ‘684 … [the final PTAB panel] would not reach a different conclusion.” The CAFC then lifted the stay on the appeal.
Prior to oral argument at the CAFC, Apple filed a post-briefing document entitled “Suggestion of Mootness.” Apple raised the issue of mootness because in a separate proceeding involving the same patents (Voip-Pal.com, Inc. v. Twitter, Inc., 798 F. App’x 644 (Fed. Cir. 2020), the CAFC affirmed that some of the claims of the patents are invalid as ineligible subject matter.
At the oral argument, Apple argued that the appeals for the overlapping claims are moot. Voip-Pal did not dispute mootness of the appeals for the overlapping claims. Since these claims were rendered invalid as ineligible subject matter in the Twitter case, the CAFC stated that appeals for these claims are rendered moot. For the remaining, 15 non-overlapping claims, Apple argued that they are essentially the same as the claims held ineligible in the Twitter case and that “basic principles of claim preclusion (res judicata) preclude Voip-Pal from accusing Apple” of infringing the non-overlapping claims in future litigation.
The CAFC stated that:
Under the doctrine of claim preclusion, “a judgment on the merits in a prior suit involving the same parties or their privies bars a second suit based on the same cause of action.” Lawlor v. Nat’l Screen Serv. Corp., 349 U.S. 322, 326 (1955). The determination of the “precise effect of the judgment[] in th[e] [first] case will necessarily have to be decided in any such later actions that may be brought.” In re Katz Interactive Call Processing Pat. Litig., 639 F.3d 1303, 1310 n.5 (Fed. Cir. 2011).
Thus, any res judicata effect of a first proceeding is “an issue that only a future court can resolve” and any preclusive effect that the Twitter case could have against the same or other parties must be decided in any subsequent action brought by Voip-Pal. The CAFC concluded that the question of obviousness for the non-overlapping claims is not moot.
Regarding the sanctions order, the CAFC stated that the final PTAB panel’s sanction order was not an abuse of discretion. Apple argued that the final PTAB panel’s sanction order violated the administrative procedure act (“APA”) because the PTAB panel issued a sanction not explicitly provided by 37 C.F.R. § 42.12(b), and thus, the PTAB panel exceeded its authority. However, the CAFC pointed out that § 42.12 provides that:
(a) The Board may impose a sanction against a party for misconduct, . . . .
(b) Sanctions include entry of one or more of the following [eight enumerated sanctions]:
The CAFC stated that because of the term “include,” the list of sanctions is non-exhaustive. The use of “may” in the rule also indicates that the PTAB has discretion to impose sanctions in the first place. Therefore, the PTAB is not limited to the eight listed sanctions in § 42.12(b). The CAFC also stated that the PTAB’s decision to allow Apple to petition for rehearing before a new panel was a reasonable course of action that provided Apple with a meaningful opportunity to respond to Voip-Pal’s letters.
Regarding Apple’s due process argument, the CAFC stated that Apple failed to identify any property interests in the course of its due process arguments, and that property interests identified for the first time on appeal are waived. An argument of a due process violation requires identification of a property interest that has been deprived. The CAFC also stated that the PTAB introduced the six letters into the record and gave Apple an opportunity to respond during the panel rehearing stage, but Apple chose not to address the substance of the letters.
Apple also argued that the PTAB erred when it determined that Apple failed to establish a motivation to combine Chu ‘684 with Chu ‘366 stating that the PTAB improperly applied the old teaching, suggestion, motivation test rather than the flexible obviousness analysis required under KSR. However, the CAFC stated that Apple’s argument was that a skilled artisan would have viewed Chu ‘684’s interface as less intuitive and less user friendly than that of Chu ‘366, and thus, a skilled artisan would have a desire to improve Chu ‘684’s system. But the PTAB determined that Apple’s expert did not provide adequate support for the proposition that a skilled artisan would have regarded Chu ‘684’s teachings as deficient. Thus, Apple failed to “articulate reasoning with some rational underpinning.” The CAFC stated that the PTAB applied the proper evidentiary standard in determining non-obviousness of the claims.
Comments
This decision shows that the PTAB has discretion in issuing sanctions and they are not limited to the list enumerated in 37 C.F.R. § 42.12. Also, issue preclusion can only be applied to a future action. The effect of a judgment in a first case must be decided in any later actions that may be brought. For demonstrating obviousness, make sure you include evidentiary support for an argument of obviousness.
The Federal Circuit Holds that an “Agreement to Agree” to License Does Not Establish an Enforceable License Right
| October 26, 2020
Phytelligence v. Washington State University
August 27, 2020
PROST, REYNA, and STOLL, Precedential opinion by Reyna
Summary
Appellant, Phytelligence, Inc. (Phytelligence) is an agricultural biotechnology company that uses tissue culture to grow trees for sale to nurseries and growers. Phytelligence entered into a Propagation Agreement with Washington State University (WSU) regarding growing of “W38” apple trees developed and patented by WSU. The Propagation Agreement was focused on research and development, but included a clause indicating that Phytelligence is “granted an option to participate” as a seller in the future upon signing “a separate contract.” Phytelligence did not execute a later contract with WSU, but later brought a legal action against WSU asserting that the Propagation Agreement granted Phytelligence a right to license. The district court affirmed WSU’s motion for summary judgment dismissing Phytelligence’s claim on the basis that the contract was an unenforceable “agreement to agree.” On appeal, the Federal Circuit affirmed the district court’s decision.
Background
Appellant, Phytelligence, Inc. (Phytelligence) is an agricultural biotechnology company that uses tissue culture to grow trees for sale to nurseries and growers.
Phytelligence engaged in discussions with Washington State University (WSU) regarding growing of “W38” apple trees developed and patented by WSU.
The parties entered into a Propagation Agreement under which Phytelligence could propagate the W38 apple trees and experiment with propagation techniques for research and development purposes. However, the agreement did not provide a right for Phytelligence to sell the W38 trees. Under the agreement, Section 4 included the following clause:
If [Phytelligence] is an authorized provider in good standing . . . by signing this Agreement, [Phytelligence] is hereby granted an option to participate as a provider and/or seller of Plant Materials listed in Exhibit A, if the Cultivar is officially released by WSU and becomes available for licensing by [WSU] . . . . [Phytelligence] will need to sign a separate contract with [WSU], or an agent of [WSU], to exercise this option.
Phytelligence emailed WSU “to clarify” that to exercise its option under Section 4, WSU would need to “grant it a separate license for the purpose of selling,” and WSU emailed “yes” in reply. Phytelligence emailed again indicating that the agreement had a “wispy forward commitment,” but then emailed stating “since this agreement is a precursor to any other, we suppose there’s no harm in going ahead and executing it. Then at least we will have the pieces in place when we are all ready to go beyond R&D mode. With that context, the agreement is fine as it is.”
In March 2013, WSU issued request for proposals seeking an exclusive licensee to manage the commercialization of WA38 and awarded an exclusive license to Proprietary Variety Management (PVM). PVM provided nonexclusive sublicenses to companies provided that such companies become Northwest Nursery Improvement Institute (NNII) member nurseries.
On May 18, 2017, Phytelligence informed WSU that it desired to proceed under Section 4 of the agreement. WSU indicated that the Propagation Agreement required a separate contract with PVM. Phytelligence approached PVM, but refused to become such a member nursery, and rejected that requirement and did not enter an agreement with PVM.
On September 15, 2017, WSU presented Phytelligence with three other options for propagating and selling WA 38, including two options that did not require NNII membership. Phytelligence rejected the three options.
On January 16, 2018, WSU terminated the Propagation Agreement with Phytelligence on the basis that Phytelligence had sold and delivered WA 38 without a license.
On February 26, 2018, Phytelligence sued WSU in state court for breach of the Propagation Agreement. WSU then asserted patent and trademark infringement counterclaims and removed the case to federal district court. WSU moved for summary judgment, arguing that Section 4 was an unenforceable “agreement to agree” under Washington state law.
The district court granted the motion for summary judgment. And, Phytelligence appealed.
The Federal Circuit’s Decision
Legal Issue
The question on appeal is whether Section 4 of the Propagation Agreement granted Phytelligence a license right as being an enforceable agreement with open terms rather than an unenforceable “agreement to agree.”
Standard of Review
The Federal Circuit reviews a grant of summary judgment under the law of the regional circuit, and, thus, de novo in this case.
Summary judgment is appropriate when “there is no genuine dispute as to any material fact and the movant is entitled to judgment as a matter of law.” Fed. R. Civ. P. 56(a).
Discussion
The Federal Circuit explained that this legal issue is a matter of contract interpretation, which is determined under state law. Under Washington state law, an “objective manifestation” standard is employed, wherein a court looks to the reasonable meaning of the contract language to determine the parties’ intent.
Agreement to Agree vs. Agreement with Open Terms
The Federal Circuit noted that an “agreement to agree” is unenforceable, explaining that “[a]n agreement to agree is an agreement to do something which requires a further meeting of the minds of the parties and without which it would not be complete.” The court explained that an “agreement with open terms” differs in that “the parties intend to be bound by … key points agreed upon with the remaining terms supplied by a court or another authoritative source, such as the Uniform Commercial Code” such that “[a]ny missing or open terms can therefore be ‘easily’ discerned by a court.” The court also explained that open terms can also be appropriate when they are based on an objective formula or method contained within the contract itself.
However, the Federal Circuit noted that Section 4 of the Propagation Agreement “provides the court with no objective method for determining the terms of the separate contract” between WSU or (or its agent).” Thus, the Federal Circuit affirmed that the Section 4 of the Propagation Agreement is merely an unenforceable agreement to agree.
Extrinsic Evidence
Phytelligence argued that the extrinsic evidence established that Section 4 was an enforceable contract with open terms. The Federal Circuit explained that under Washington law, to assist in determining the meaning of contract language, a “context rule” is also applied that includes “examination of the context surrounding a contract’s execution, including the consideration of extrinsic evidence.” However, the court explained that extrinsic evidence is to only be used “to determine the meaning of specific words and terms used and not to show an intention independent of the instrument …”
Moreover, the Federal Circuit explained that extrinsic evidence revealed that no agreement was reached and pointed out that in emails to WSU, Phytelligence actually acknowledged that Section 4 contained a “wispy forward commitment” and that there was “no harm” to sign the agreement because the agreement “is a precursor to any other.” The Federal Circuit noted that “[o]n these undisputed material facts, no reasonable fact finder could conclude that at the time of execution, Phytelligence and WSU agreed” that Section 4 would contain the terms of the later PVM form license, but rather that WSU did not commit to any definite terms of a future license.
Phytelligence also argued that a declaration of the CEO of Phytelligence supported that there was a material dispute because the CEO had declared “My understanding … was that … Phytelligence would have the option to acquire a license on the standard terms.” However, the court expressed that “mere allegation and speculation do not create a factual dispute for purposes of summary judgment.”
Phytelligence also argued that the parties’ conduct after the execution of the Propagation Agreement also created a factual dispute. However, the court rejected that argument and noted that WSU had offered Phytelligence three different licensing options, including two that didn’t require joining of NNII, which were all rejected by Phytelligence. The court explained that “[b]ased on this evidence, no reasonable fact finder could conclude that, at the time of execution, the parties understood that there was nothing left for future negotiation regarding the terms of Phytelligence’s separate contact under Section 4.”
Accordingly, the Federal Circuit affirmed the district court’s decision.
Takeaways
- In drafting agreements, it is important to keep in mind that an “agreement to agree” at some later date regarding any terms of the agreement is not enforceable. Accordingly, one should exercise care to avoid development of contract terms of this nature.
- In drafting agreements, it is also important to appreciate that some contract terms can be drafted as “open terms” that may still be enforceable, but that for such “open terms” to be enforceable, such “open terms” must be defined in the contract in a manner to be supplied by a court or another authoritative source or based on some objective formula or method set forth in the contract itself.
- In collaborative relationships, it is also important to carefully plan for future circumstances. In this case, Phytelligence’s emails prior to signing of the Propagation Agreement reflected a concern that there was no agreement for future sale of the product. However, rather than effectively addressing the issue at that time, Phytelligence moved forward with the relationship regarding research and development without sufficiently securing its future ability to engage in sale of the product.
Tags: Agreement to Agreement > contract > Intent of Parties > interpretation > license
Same difference: A broad interpretation of “contrast” renders patented design obvious in view of prior art showing a similar “level of contrast”.
| October 19, 2020
Sealy Technology, LLC v. SSB Manufacturing Company (non-precedential)
August 26, 2020
Before Prost, Reyna, and Hughes (Opinion by Prost).
Summary
In nixing a design patent purporting to show “contrast” between design elements, the Federal Circuit broadly interprets “contrast” in the claimed design as not requiring a level of contrast that is different than what is available in the prior art.
Details
In their briefs to the Federal Circuit, Sealy Technology, LLC (“Sealy”) told the story about how, during a slump in sales around 2009, they re-designed their Stearns & Foster luxury-brand mattresses to visually distinguish their mattresses in a marketplace overrun with white or otherwise monochromatic mattress designs.
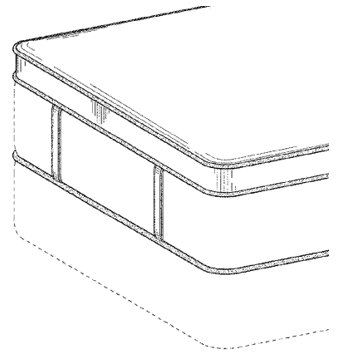
To be visually distinct, Sealy incorporated a “bold” combination of contrast edging running horizontally along the edges of the mattress and around the handles on the mattress.

Sealy claimed this design in their U.S. Design Patent No. 622,531 (“D531 patent”). The D531 patent claimed “[t]he ornamental design for a Euro-top mattress design, as shown and described”, with Figure 1 being representative:
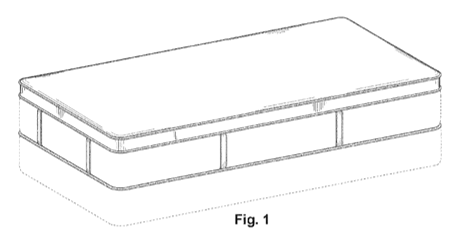
The D531 patent did not contain any textual descriptions of the contrast edging in Sealy’s mattress design.
Sealy’s dispute with Simmons Bedding Company (“Simmons”) began shortly after the D531 patent issued, when in 2010, Sealy sued Simmons for infringing the D531 patent. The product at issue was Simmons’s Beautyrest-brand mattress:

in 2011, Simmons sought an inter partes reexamination of the D531 patent. During the first round of the reexamination, the Examiner interpreted the claimed design as including the following elements:
- The horizontal piping along the edges of the top and bottom of the mattress and along the top flat surface of the pillow top layer has a contrasting appearance.
- The [eight] flat vertical handles each have contrasting sides of edges running vertically with the length of the handles.
However, the Examiner declined to adopt any of Simmons’s requested anticipation and obviousness challenges. And when Simmons appealed the Examiner’s refusal to the Patent Trial and Appeal Board, Sealy vouched for the Examiner’s interpretation.
Unfortunately for Sealy, the Board on appeal entered new obviousness rejections. A second round of the reexamination followed. This time around, the Examiner adopted the Board’s rejections, which Sealy was unable to overcome even after a second appeal to the Board. Sealy then appealed the rejections to the Federal Circuit.
Sealy raised two main issues on appeal: first, whether the Board correctly construed the “contrast” in the claimed design; and second, whether the cited prior art was a proper primary reference.
On the question of claim construction, the Board had determined that “the only contrast necessary is one of differing appearance from the rest of the mattress”, whether by contrasting fabric, contrasting color, contrasting pattern, and contrasting texture.
Sealy argued that the Board’s construction was too broad. The proper construction, argued Sealy, should require a more specific level of contrast, that is, “a contrasting value and/or color” or “something that causes the edge to stand out or to be strikingly different and distinct from the rest of the design”.
Sealy relied on MPEP 1503.02(II), which provides that “contrast in materials may be shown by using line shading in one area and stippling in another”, that “the claim will broadly cover contrasting surfaces unlimited by color”, and that “the claim would not be limited to specific material”. Sealy also argued that a designer of ordinary skill in the art would have understood the D531 patent as requiring a more specific degree of contrast than the Board’s construction.
The Federal Circuit disagreed.
The Federal Circuit agreed with the Examiner and the Board’s findings that the patent contained no textual descriptions about contrast in the claimed design, that the drawings did not convey a different level of contrast than the prior art, and that Sealy’s argument regarding the understanding of a designer skilled in the art lacked evidentiary support..
On the question of obviousness, Sealy challenged the Board’s reliance on the Aireloom Heritage mattress shown below, arguing that the prior art mattress lacked the requisite contrast.

The weakness in Sealy’s argument was that its success depended entirely on the Federal Circuit adopting Sealy’s claim construction. And because the Federal Circuit did not find that the claimed design required a “strikingly different” contrast between the edging and the rest of the mattress, the Federal Circuit agreed with the Board’s determination that the prior art mattress had “at least some difference in appearance” between the edging and the rest of the mattress. This was sufficient to qualify the Aireloom Heritage mattress as a proper primary reference.
Takeaway
- Consider adding strategic textual descriptions in the specification to clarify the claimed design. For example, in Sealy’s case, since the contrast in the claimed design is provided in part by contrast in color, it may have been helpful to include such descriptions as “Figure 1 is a perspective view of a mattress showing a first embodiment f the Euro-top mattress design with portions shown in stippling to indicate color contrast”.
- If color contrast is important to the claimed design, consider adding solid black shading to the appropriate portions. 37 C.F.R. 1.152 (“Solid black surface shading is not permitted except when used to represent the color black as well as color contrast”).
- If the color scheme is important to the claimed design, consider filing a color drawing with the design application. One of Apple’s design patents on the iPhone graphical user interface includes a color drawing, which helped Apple’s infringement case against Samsung because similarities between the claimed design and the graphical user interface on Samsung’s Galaxy phones were immediately noticeable.
LICENSING AGREEMENTS AND TESTIMONIES COULD BE USED AS EVIDENCE OF SECONDARY CONSIDERATIONS TO OVERCOME OBVIOUSNESS ONLY WHEN THEY PROVIDE A “NEXUS” TO THE PATENTS
| October 10, 2020
Siemens Mobility, Inc. v. U.S. PTO
September 8, 2020
Lourie (author), Moore, and O’Malley
Summary:
The Federal Circuit affirmed the PTAB’s final decision that claims of Siemens’s patents are unpatentable as obvious. The Federal Circuit found that the PTAB’s findings in claim construction of “corresponding regulations,” and evaluation of Siemens’s evidence of secondary considerations are clearly supported by substantial evidence.
Details:
Siemens Mobility, Inc. (“Siemens”) appeals from two final decisions of the PTAB, where the PTAB held that claims 1-9 and 11-19 of U.S. Patent No. 6,609,049 (“the ‘049 patent) and claims 1-9 and 11-19 of U.S. Patent No. 6,824,110 (“the ‘110 patent) were unpatentable.
The ‘049 patent and ‘110 patent
Siemens’s ‘049 and ‘110 patents are directed to methods and systems for automatically activating a train warning device, including a horn, at various locations. The systems include a control unit, a GPS receiver, and database of locations of grade crossings, and a horn. Siemens’s two patent disclose that if that grade crossing is subject to state regulations, the horn is activated based on those state regulations. If that grade crossing is not subject to state regulations, Siemens’s system considers that crossing as subject to a Federal Railroad Administration regulation and sounds the horn when the train is 24 seconds or fewer away from the crossing.
Independent claim 1 of ‘110 patent:
1. A computerized method for activating a warning device on a train at a location comprising the steps of:
maintaining a database of locations at which the warning device must be activated
and corresponding regulations concerning activation of the warning device;
obtaining a position of the train from a positioning system;
selecting a next upcoming location from among the locations in the database based at least in part on the position;
determining a point at which to activate the warning device in compliance with a
regulation corresponding to the next upcoming location; and
activating the warning device at the point.
The claims of the ‘049 and ‘110 patents substantially similar.
PTAB
Westinghouse Air Brake Technologies Corporation (“Westinghouse”) petitioned for IPR, challenging claims of both ‘049 and ‘110 patents under §103.
The PTAB found that all challenged claims would have been obvious over two references (Byers and Michalek). In addition, the PTAB did not found Siemens’s evidence of secondary considerations to be persuasive. Finally, the PTAB found that a skilled artisan would have combined the teachings of both references.
Siemens appealed.
CAFC
Three aspects of the PTAB decisions at issue in the appeal:
- The board’s claim construction of “corresponding regulations”;
- The board’s evaluation of Siemens’s evidence of secondary considerations; and
- The board’s findings of a person of skill in the art would have combined both references.
As for the second issue, Siemens presented two license agreements to both patents. Also, Siemens provided evidence regarding Westinghouse’s request to license and testimony from Westinghouse employees regarding the strength of two patents.
Siemens argued that the PTAB improperly discounted this evidence for lack of nexus.
The CAFC did not find those license agreements to be persuasive.
The CAFC noted that the license agreement with Norfolk Southern was presented to the PTAB with royalty information redacted and that another license was provided only for a nominal fee. Also, the CAFC noted that a license request from Westinghouse was for avoiding the cost of a pending patent infringement suit.
Furthermore, the CAFC agreed with the PTAB’s position that testimony from Westinghouse “provided a scant basis for accessing the value of the ‘110 patent” because while the testimony referred to a “horn sequencing patent” or “automatic horn activation,” it did not provide any connection to the language of the claims.
Therefore, the CAFC held that the PTAB’s findings were clearly supported by substantial evidence.
Takeaway:
- In the obviousness analysis, a nexus is required between the merits of the claimed invention and the offered evidence.
- Licensing agreements and testimonies could be used as evidence of secondary considerations to overcome obviousness only when they provide a “nexus” to the patents at issue.
Tags: employee testimony > evidence of secondary considerations > licensing agreement > nexus > obviousness
Prosecution history estoppel applies to narrowing amendment for a purpose, but not for all purposes
| September 25, 2020
Bio-Rad Laboratories, Inc., Et Al. v. 10X Genomics Inc.
August 3, 2020
Newman, O’Malley (Opinion author), and Taranto
Summary
The Federal Circuit held that 10X infringes Bio-Rad’s ‘083 patent under the doctrine of equivalents because a tangential exception to the prosecution history estoppel applies in this case.
Details
Bio-Rad Laboratories, Inc. and the University of Chicago (collectively, “Bio-Rad”) accused 10X Genomics Inc. (“10X”) of infringing three patents: U.S. Patent Nos. 8,888,083 (“083 patent”); 8,304,193 (“193 patent”); and 8,329,407 (“407 patent”). The United States District Court for the District of Delaware held that 10X willfully infringed all three patents and awarded damages in an amount of $23,930,716.
The patents-in-suit are directed to systems and methods for forming microscopic droplets (also called “plugs”) of fluids to perform biochemical reactions. Claim 1 of the ‘083 patent, as below, is representative:
A microfluidic system comprising:
a non-fluorinated microchannel;
a carrier fluid comprising a fluorinated oil and a fluorinated surfactant comprising a hydrophilic head group in the microchannel;
at least one plug comprising an aqueous plug-fluid in the microchannel and substantially encased by the carrier-fluid, wherein the fluorinated surfactant is present at a concentration such that surface tension at the plug-fluid/microchannel wall interface is higher than surface tension at the plug-fluid/carrier fluid interface.
During prosecution, the inventors amended the claims, as underlined above, to distinguish from a prior art, U.S. Patent No. 7,294,503 (“Quake”). Quake disclosed microchannels formed or coated with Teflon (a fluorinated polymer) or other fluorinated oils. Furthermore, the inventors argued that their invention attempts to prevent droplets from sticking to the walls of microchannels and requires that the “surfactant should be chemically similar to the carrier fluid and chemically different from the channel walls.” That is, the non-fluorinated microchannels and the fluorinated surfactant are required not to react with each other. This is the purpose of the amendment. In contrast, Quake did not teach that microchannels and carriers fluids were chemically distinct, and the fluorinated microchannels and surfactants could, therefore, react with each other.
After the litigation was filed, 10X modified its products to add 0.02% Kynar-a non-reactive amount of a fluorine-containing resin-to its microchannels. 10X concedes that the addition of this amount of Kynar is irrelevant to the functioning of its products. In the District Court, the jury found that 10X’s accused products, as modified, do not literally satisfy the “non-fluorinated microchannels” limitation, but meet the limitation under the doctrine of equivalents. On appeal, among other issues, 10X argued that prosecution history estoppel and claim vitiation barred Bio-Rad’s theory of equivalence. The District Court held that prosecution history estoppel does not apply in this case because the amendment at issue was only tangentially related to the accused equivalent.
On appeal, 10X continued to argue that prosecution history estoppel applies because the inventors’ amendment narrowed the claims to recite a “non-fluorinated microchannel” to overcome Quake, which taught “fluorinated” microchannels. As such, the inventors surrendered the right to expand their monopoly to cover microchannels containing fluorine, “for whatever purpose.” In response, Bio-Rad counter argued that the reason for narrowing the claims was peripheral, or not directly relevant to the alleged equivalent. Bio-Rad contends that the patentees amended the claims to make clear that the carrier fluid and the microchannel wall should be chemically distinct, which bears no more than a tangential relation to the alleged equivalent-microchannel walls containing a nominal amount of fluorine that is not chemically distinct from the carrier fluid. The Federal Circuit agreed with Bio-Rad, and reasoned that inventors surrendered microchannels coated with fluorine that reacted with carrier fluids, not those containing de minimis amounts of fluorine that have no effect on how the microchannel functions in the system. The narrowing amendment can only be said to have a tangential relation to the equivalent at issue-negligibly fluorinated microchannels, or microchannels with non-fluorinated properties. That is, the inventors surrendered microchannels coated with fluorine for a purpose, but not for all purposes.
Take away
- During prosecution, inventors should also explain in detail the purpose of the amendment of claims in order to narrow the scope surrendered by the amendment.
Tags: doctrine of equivalents > prosecution history estoppel > tangential exception
Be careful in translating foreign applications into English, particularly with respect to uncommon terms
| September 18, 2020
IBSA Institut Biochimique, S.A., Altergon, S.A., IBSA Pharma Inc. v. Teva Pharmaceuticals USA, Inc.
July 31, 2020
Prost (Chief Judge), Reyna, and Hughes. Opinion by Prost.
Summary
In a U.S. application claiming priority to an Italian application, the term “semiliquido” was translated as “half-liquid.” This term was found to be indefinite, in view of a lack of a clear meaning in the art, as well as the statements of the prosecution history and specification. Further, attempts to argue that the term “half-liquid” should be interpreted as “semi-liquid” failed due to inconsistencies with the specification.
Details
IBSA is the owner of U.S. Patent No. 7,723,390, which claims priority to an Italian application. Claim 1 of the ‘390 patent is as follows:
1. A pharmaceutical composition comprising thyroid hormones or their sodium salts in the form of either:
a) a soft elastic capsule consisting of a shell of gelatin material containing a liquid or half-liquid inner phase comprising said thyroid hormones or their salts in a range between 0.001 and 1% by weight of said inner phase, dissolved in gelatin and/or glycerol, and optionally ethanol, said liquid or half-liquid inner phase being in direct contact with said shell without any interposed layers, or
b) a swallowable uniform soft-gel matrix comprising glycerol and said thyroid hormones or their salts in a range between 0.001 and 1% by weight of said matrix.
Teva filed an ANDA with a Paragraph IV certification asserting that the patent is invalid. In particular, Teva asserted that claim 1 is invalid as being indefinite. Upon filing of the ANDA, IBSA filed suit against Teva.
The key issue in this case is the meaning of the term “half-liquid.” IBSA asserted that this term should mean “semi-liquid, i.e., having a thick consistency between solid and liquid.” Meanwhile, Teva argued that the term is either invalid, or should be interpreted as a “non-solid, non-paste, non-gel, non-slurry, non-gas substance.”
District Court
At the district court, all parties agreed that the intrinsic record does not define the term “half-liquid.” However, IBSA asserted that its construction is supported by intrinsic evidence, including the Italian priority application. The Italian priority application used the term “semiliquido” at all places where the ‘390 patent used the term “half-liquid.” In 2019, IBSA obtained a certified translation in which “semiliquido” was translated as “semi-liquid.” IBSA asserted that “semi-liquid” and “half-liquid” would be understood as synonyms.
However, the district court noted that there were other differences between the Italian priority application and the ‘390 patent specification, beyond the “semiliquido”/“half-liquid” difference. These differences included the “Field of Invention” and “Prior Art” sections. Because of these other differences, the district court concluded that the terminology in the U.S. specification reflected the Applicant’s intent, and that any difference was deliberate.
Furthermore, the district court noted that during prosecution, there was at one point a dependent claim using the term “semi-liquid” which was dependent on an independent claim using the term “half-liquid.” Even though the term “semi-liquid” was eventually deleted, this was interpreted as evidence that the terms are not synonymous.
Additionally, the specification cited to pharmaceutical references that used the term “semi-liquid.” According to the district court, this shows that the Applicant knew of the term “semi-liquid,” but chose to use the term “half-liquid” instead.
Finally, all extrinsic evidence was considered by the district court to be unpersuasive. The extrinsic evidence used the term “half-liquid” in a different context than the present application. As such, the district court concluded that the term “half-liquid” was “exceedingly unlikely” to be a term of art at the time of filing.
Next, the district court analyzed whether a skilled artisan would nevertheless be able to determine the meaning of the term “half-liquid.” First, the court noted that the claims do not clarify what qualifies as a half liquid, other than the fact that it is neither liquid nor solid. The district court concluded that one skilled in the art would only understand “half-liquid” to be something that is not a gel or paste. This conclusion was based on a passage in the specification that stated:
In particular, said soft capsule contains an inner phase consisting of a liquid, half-liquid, a paste, a gel, an emulsion, or a suspension comprising the liquid (or half-liquid) vehicle and the thyroid hormones together with possible excipients in suspension or solution.
Additionally, during prosecution, the Applicant responded to an obviousness rejection by including the statement that the claimed invention “is not a macromolecular gel-lattice matrix” or a “high concentration slurry.” As such, the Applicant disclaimed these from the scope of “half-liquid.”
Finally, the district court commented on the expert testimony. IBSA’s expert had “difficulty articulating the boundaries of ‘half-liquid’” during his deposition. Meanwhile, Teva’s expert stated that “half-liquid is not a well-known term in the art.”
Based on the above, the district court concluded that one having ordinary skill in the art would find it impossible to know “whether they are dealing with a half-liquid within the meaning of the claim,” and concluded that the claims are invalid as being indefinite.
CAFC
The CAFC agreed with the district court on all points. First, the CAFC agreed that the claim language does not clarify the issue. Rather, the claim language only clarifies that “half-liquid” is not the same as liquid.
As to the specification, the CAFC agreed with the district court that the portion of the specification noted above clarifies that “half-liquid” cannot mean a gel or paste. Moreover, the CAFC cited another passage, which refers to “soft capsules (SEC) with liquid, half-liquid, paste-like or gel-like inner phase.” The CAFC concluded that the use of the word “or” in these passages shows that “half-liquid” is an alternative to the other members of the list, such as pastes and gels. Meanwhile, since gels and pastes have a thick consistency, IBSA’s proposed claim construction is in contradiction with these passages of the specification.
Additionally, the CAFC refuted IBSA’s argument that the specification includes passages that are at odds with the description of “half-liquid” as an alternative to pastes and gels. These passages include:
…an SEC capsule containing an inner phase consisting of a paste or gel comprising gelatin and thyroid hormones or pharmaceutically acceptable salts thereof…in a liquid or half liquid.
However, this argument conflates the inner phase and the vehicle within the inner phase, and does not explain why these are the same.
Additionally, the CAFC noted that the specification includes a list of examples of liquid or half-liquid vehicles, and a reference to a primer on making “semi-liquids.” However, even if some portions of the specification could support the position that “half-liquid” and “semi-liquid” are synonyms, the specification fails to describe the boundaries of “half-liquid.”
Next, the CAFC discussed the prosecution history. As in the district court, IBSA asserted that “half-liquid” means “semi-liquid.” This was because the term “half-liquid” was used in all places where “semiliquido” was used, and a certified translation showed that “semiliquido” is translated as “semi-liquid.”
The CAFC agreed the use of “half-liquid” was intentional, relying on not only the differences between the Italian application and the U.S. application noted by the district court, but others as well. In particular, claim 1 of the U.S. application differed from claim 1 of the Italian application, and incorporated an embodiment not found in the Italian application. Further, the Italian application does not use the term “gel” in the first passage noted above.
The CAFC also agreed with the district court that the temporary presence of a claim reciting “semi-liquid” dependent on a claim reciting “half-liquid” further demonstrates that these terms are not synonymous.
Finally, the CAFC reviewed the extrinsic evidence. The CAFC noted the IBSA’s expert’s inability to explain the boundaries of the term “half-liquid.” The CAFC also pointed out that only one dictionary of record had a definition including the term “half liquid,” and this was a non-scientific dictionary. This dictionary defined “semi-liquid” as “Half liquid; semifluid.” But, IBSA’s expert stated that “semifluid” and “half-liquid” are not necessarily the same.
Further, although other patents using the term “half-liquid” were cited, these patents used the term in a different context, and thus were not helpful to define the term in the context of the ‘390 patent. IBSA’s expert acknowledged the lack of any other literature such a textbook or a peer-reviewed journal using the term “half-liquid.” Additionally, IBSA’s expert stated that he was uncertain about whether his construction of “half-liquid” would exclude gels or slurries.
Based on the above, the CAFC concluded that the term “half-liquid” does not have a definite meaning. As such, the claim is invalid as failing to comply with §112.
Takeaways
-When translating a specification from a foreign language to English, it is preferable that the specifications are as close to identical as possible.
-If there are differences between a foreign language specification and the U.S. specification, these differences should ideally be limited to non-technical portions of the specification.
-If using a term that is not widely used, it is recommended to clearly define this term in the record. Failure to do so may result in the term being interpreted in an unintended manner, or worse, the term being considered indefinite.
-Where there are discrepancies between a foreign priority application and a US application, such differences may be interpreted as intentional.
In a patent infringement action involving standard essential patents, standard-essentiality is to be determined by judges in bench trials and juries in jury trials
| September 11, 2020
Godo Kaisha IP Bridge 1 v. TCL Communication Technology Holdings Ltd. (Fed. Cir. 2020) (Case No. 2019-2215)
August 4, 2020
Prost (Chief Judge), Newman and O’Malley. Court opinion by O’Malley
Summary
The Federal Circuit unanimously affirmed all aspects of the district court’s rulings and the verdict predicated thereon in favor of a patentee in a patent infringement action involving standard essential patents. In Fujitsu, the Court endorsed standard compliance as a way of proving infringement, holding that, if a district court construes the claims and finds that the reach of the claims includes any device that practices a standard, then this can be sufficient for a finding of infringement. In this case, the Court moved on to hold that standard-essentiality is a question for the factfinder (meaning judges in bench trials and juries in jury trials), rejecting the defendant’s argument that, under Fujitsu, the court must first make a threshold determination as part of claim construction that all implementations of a standard infringe the claims.
Details
I. background
In a patent infringement action for U.S. Patent Nos. 8,385,239 and 8,351,538 in the U.S. District Court for the District of Delaware filed by Godo Kaisha IP Bridge 1 (“IP Bridge”) against TCL Communication Technology Holdings Ltd. et al. (“TCL”), asserted claims are directed to the Long-Term Evolution (“LTE”) standard, a standard for wireless broadband communication for mobile devices and data terminals, and IP Bridge’s infringement theory relied on Fujitsu Ltd. v. Netgear Inc., 620 F.3d 1321 (Fed. Cir. 2010), holding, on appeal from a summary judgment decision, that a district court may rely on an industry standard in analyzing infringement. Specifically, the Federal Circuit stated:
“We hold that a district court may rely on an industry standard in analyzing infringement. If a district court construes the claims and finds that the reach of the claims includes any device that practices a standard, then this can be sufficient for a finding of infringement. We agree that claims should be compared to the accused product to determine infringement. However, if an accused product operates in accordance with a standard, then comparing the claims to that standard is the same as comparing the claims to the accused product. We accepted this approach in [Dynacore Holdings Corp. v. U.S. Philips Corp., 363 F.3d 1263 (Fed.Cir.2004)] where the court held a claim not infringed by comparing it to an industry standard rather than an accused product. An accused infringer is free to either prove that the claims do not cover all implementations of the standard or to prove that it does not practice the standard.” Fujitsu, 620 F.3d at 1327.
IP Bridge presented evidence to show that (1) the asserted claims are essential to mandatory sections of the LTE standard; and (2) the accused products comply with the LTE standard. In contrast, TCL did not present any evidence to counter that showing.
After a jury trial in 2018, the jury found that TCL was liable for infringement of the asserted claims by its sale of LTE standard-compliant devices such as mobile phones and tablets. The jury also awarded IP Bridge damages in the amount of $950,000.
Following the verdict, TCL filed a motion for judgment as a matter of law (“JMOL”), contending that IP Bridge’s theory of infringement was flawed because the Fujitsu “narrow exception” to proving infringement in the standard way, and IP Bridge filed a motion to amend the judgment under Federal Rule of Civil Procedure 59(e), seeking supplemental damages and an accounting of infringing sales of all adjudicated products through the date of the verdict, and ongoing royalties for TCL’s LTE standard-compliant products, “both adjudicated and non-adjudicated.”
The district court denied TCL’s JMOL motion, concluding that substantial evidence supported the jury’s infringement verdict. In contrast, the district court awarded the requested pre-verdict supplemental damages, and awarded on-going royalties in that amount for both the adjudicated products and certain unadjudicated products, with finding that the jury’s award represented a FRAND (Fair, Reasonable And Non-Discriminatory) royalty rate of $0.04 per patent per infringing product. The district court reasoned that, because IP Bridge demonstrated at trial that LTE standard-compliant devices do not operate on the LTE network without infringing the asserted claims, the unaccused, unadjudicated products “are not colorably different tha[n] the accused products.”
TCL timely appealed the district court’s infringement finding and its rulings regarding royalties.
II. The Federal Circuit
The Federal Circuit unanimously affirmed all aspects of the district court’s rulings and the verdict predicated thereon in favor of a patentee in a patent infringement action involving standard essential patents.
The issue in the appeal was a question not expressly answered by its case law including Fujitsu: who determines the standard-essentiality of the patent claims at issue—the court, as part of claim construction, or the jury, as part of its infringement analysis? The Court agreed with IP Bridge that standard-essentiality is a question for the factfinder.
TCL’s entire appeal rested on a single statement from Fujitsu, 620 F.3d at 1327: “If a district court construes the claims and finds that the reach of the claims includes any device that practices a standard, then this can be sufficient for a finding of infringement” (emphasis added). In TCL’s view, the statement mandates that a district court must first determine, as a matter of law and as part of claim construction, that the scope of the claims includes any device that practices the standard at issue.
The Court rejected TCL’s contention for three reasons below.
First, the Court pointed out that the statement in Fujitsu assumed the absence of genuine disputes of fact on the two steps of that analysis, which would be necessary to resolve the question at the summary judgment stage. The passing reference in Fujitsu to claim construction is simply a recognition of the fact that the first step in any infringement analysis is claim construction.
Second, the Court stated that its reading of Fujitsu is buttressed by that decision’s reference to Dynacore where the Court affirmed the summary judgment of non-infringement because the patentee did not show that a particular claim limitation was mandatory in the standard. The Court read Dynacore such that: “Although we referenced the claim construction by which the patentee was bound, Dynacore considered the possibility of the dispute going to the jury and rejected it based on undisputed facts.” Based on this, the Court concluded that, under Dynacore, which Fujitsu referenced in its holding, standard-essentiality of patent claims is a fact issue. The Court noted that the standard-essentiality may be amenable to resolution on summary judgment in appropriate cases like any other fact issue, but that it does not mean it becomes a question of law.
Finally, the Court expressed a view that determining standard-essentiality of patent claims during claim construction hardly makes sense from a practical point of view. Essentiality is, after all, a fact question about whether the claim elements read onto mandatory portions of a standard that standard-compliant devices must incorporate. In the Court’s view, this inquiry is more akin to an infringement analysis (comparing claim elements to an accused product) than to a claim construction analysis (focusing, to a large degree, on intrinsic evidence and saying what the claims mean).
From the foregoing, the Court found that substantial evidence fully supports the jury’s infringement verdict.
Further, the Court saw no reason to disturb the district court’s conclusions after carefully considering TCL’s remaining arguments—including its argument that the district court abused its discretion in awarding on-going royalties in this case.
In conclusion, the Court unanimously affirmed all aspects of the district court’s rulings and the verdict predicated thereon in favor of a patentee in a patent infringement action involving standard essential patents.
Takeaway
· In a patent infringement action involving standard essential patents, standard-essentiality is to be determined by judges in bench trials and juries in jury trials.
Proprietary interest is not required in seeking cancellation of a trademark registration
| August 31, 2020
Australian Therapeutic Supplies Pty. Ltd. v. Naked TM, LLC
July 27, 2020
O’Malley, Reyna, Wallach (Opinion by Reyna; Dissent by Wallach)
Summary
The Trademark Trial and Appeal Board (the “TTAB”) determined that Australian Therapeutic Supplies Pty. Ltd. (“Australian”) lacked standing to bring a cancellation proceeding against a trademark registration of Naked TM, LLC (“Naked”), because Australian lacked proprietary rights in its unregistered marks. The United States Court of Appeals for the Federal Circuit (the “CAFC”) reversed and remanded, holding that proprietary interest is not required in seeking cancellation of a trademark registration. By demonstrating real interest in the cancellation proceeding and a reasonable belief of damage, statutory requirement to bring a cancellation proceeding under 15 U.S.C. § 1064 is satisfied.
Details
Australian started using the mark NAKED and NAKED CONDOM for condoms in Australia in early 2000. Australian then began advertising, selling, and shipping the goods bearing the marks to customers in the United States starting as early as April 2003.
Naked owns U.S. Trademark Registration No. 3,325,577 for the mark NAKED for condoms. In 2005, Australian became aware of the trademark application filed on September 22, 2003 by Naked’s predecessor-in-interest. On July 26, 2006, Australian contacted Naked, claiming its rights in its unregistered mark. From July 26, 2006 to early 2007, Australian and Naked engaged in settlement negotiations over email. Naked asserts that the email communications resulted in a settlement, whereby Australian would discontinue use of its unregistered mark in the United States, and Australian consents to Naked’s use and registration of its NAKED mark in the United States. Australian asserts the parties failed to agree on the final terms of a settlement, and no agreement exists.
In 2006, Australian filed a petition to cancel registration of he NAKED mark, asserting Australian’s prior use of the mark, seeking cancellation on the grounds of fraud, likelihood of confusion, false suggestion of a connection, and lack of bona fide intent to use the mark. Naked responded, denying the allegations and asserting affirmative defenses, one of which was that Australian lacked standing, as Australian was contractually and equitably estopped from pursuing the cancellation.
Following trial, on December 21, 2018, the Board concluded that Australian lacked standing to bring the cancellation proceeding, reasoning that Australian failed to establish proprietary rights in its unregistered mark and therefore, lacked standing. The Board found that while no formal written agreement existed, through email communications and parties’ actions, the Board found that Australian led Naked to reasonably believe that Australian had abandoned its rights to the NAKED mark in the United States in connection with condoms. While the Board made no finding on whether Australian agreed not to challenge Naked’s use and registration of the NAKED mark, the Board concluded that Australian lacked standing because it could not establish real interest in the cancellation or a reasonably basis to believe it would suffer damage from Naked’s continued registration of the mark NAKED.
The statutory requirements to bring a cancellation proceeding under 15 U.S.C. § 1064 are 1) demonstration of a real interest in the proceeding; and 2) a reasonable belief of damage. The CAFC held that the Board erred by concluding that Australian lacked standing because it had no proprietary rights in its unregistered mark. Australian contracting away its rights to use the NAKED mark in the United States could bar Australian from proving actual damage, the CAFC clarified that 15 U.S.C. § 1064 requires only a belief of damage. In sum, establishing proprietary rights is not a requirement for demonstrating a real interest in the proceeding and a belief of damage.
Next, the CAFC considered whether Australian has a real interest and reasonable belief of damage such that is has a cause of action under 15 U.S.C. § 1064. Here, Australian demonstrates a real interest because it had twice attempted to register its mark in 2005 and 2012, but was refused registration based on a likelihood of confusion with Naked’s registered mark. The USPTO has suspended prosecution of Australian’s later-filed application, pending termination of the cancellation proceeding, which further demonstrates a belief of damage.
Naked argued that Australian’s applications do not support a cause of action because Australian abandoned its first application. It also argued that ownership of a pending application does not provide standing.
With regard to the first point, the CAFC stated that abandoning prosecution does not signify abandoning of its rights in a mark. As for the second point, Australian’s advertising and sales in the United States since April 2003, supported by substantial evidence, demonstrate a real interest and reasonable belief of damage.
While Naked questions the sufficiency of Australian’s commercial activity, the CAFC stated that minimum threshold of commercial activity is not imposed by 15 U.S.C. § 1064.
Therefore, the CAFC held that the Board erred by requiring proprietary rights in order to establish a cause of action under 15 U.S.C. § 1064. The CAFC also held that based on the facts before the Board, Australian had real interest and a reasonable belief of damage; the statutory requirements for seeking a cancellation proceeding is thereby satisfied. The CAFC reversed and remanded the case to the Board for further proceedings.
Dissenting Opinion
While Judge Wallach, in his dissenting opinion agreed that proprietary interest is not required, he disagreed with the majority’s finding that Australian met its burden of proving a real interest and a reasonable belief in damages. In this case, any “legitimate commercial interest” in the NAKED mark was contracted away, as was any “reasonable belief in damages”.
Takeaway
Proprietary interest is not required in seeking cancellation of a trademark registration. Statutory requirement to bring a cancellation proceeding under 15 U.S.C. § 1064 is satisfied by demonstrating real interest in the cancellation proceeding and a reasonable belief of damage.
PTAB Should Provide Adequate Reasoning and Evidence in Its Decision and Teaching Away Argument Requires More Than a General Preference
| August 26, 2020
ALACRITECH, INC. V. INTEL CORP., CAVIUM, LLC, DELL, INC.
July 31, 2020
Before Stoll, Chen, and Moore
Summary
The Federal Circuit reviewed the adequacy of the Patent Trial and Appeal Board’s (Board) obviousness findings under the Administrative Procedure Act’s (APA) requirement that the Board’s decisions be supported by adequate reasoning and evidence. The Federal Circuit agreed with Alacritech’s argument that the Board did not adequately support the finding. The Federal Circuit found no reversible error in the Board’s remaining obviousness determination. The Federal Circuit vacated in part and remanded the Board’s decision.
Background
Alacritech, Inc. owns the patent 8,131,880. Intel Corp., et al., (Intel) petitioned to the Board for inter partes review (IPR) of the patent ‘880. The Board’s final decisions found the challenged claims unpatentable as obvious. Alacritech appealed the Board’s obviousness decision for four independent claims.
The ‘880 patent relates to computer networking and is directed to a network-related apparatus and method for offloading certain network processing from a central processing unit (CPU) to an “intelligent network interface card.” Intel asserted that the challenged claims would have been obvious over Thia1 in view of Tanenbaum2. The Board agreed and held all of the challenged claims unpatentable as obvious in the final written decisions. On appeal in the Federal Circuit, one of the focus was on claim 41 reciting “a flow re-assembler disposed in the network interface” as follows:
| 41. An apparatus for transferring a packet to a host computer system, comprising: a traffic classifier, disposed in a network interface for the host computer system, configured to classify a first packet received from a network by a communication flow that includes said first packet; a packet memory, disposed in the network interface, configured to store said first packet; a packet batching module, disposed in the network interface, configured to determine whether another packet in said packet memory belongs to said communication flow; a flow re-assembler, disposed in the network interface, configured to re-assemble a data portion of said first packet with a data portion of a second packet in said communication flow; and a processor, disposed in the network interface, that maintains a TCP connection for the communication flow, the TCP connection stored as a control block on the network interface. |
Discussion
Alacritech argued that the Board’s analysis was inadequate to support its findings on claims 41-43. Independent claim 43 is similar to claim 41, but recites a limitation in the network interface with “a re-assembler for storing data portions of said multiple packets without header portions in a first portion of said memory.” The dispute was focused on where reassembly takes place in the prior art and whether that location satisfies the claim limitations. The alleged claims incorporate limitations that require reassembly in the network interface, as opposed to a central processor. Intel argued that “Thia . . . discloses a flow re-assembler on the network interface to re-assemble data portions of packets within a communication flow.” In response, Alacritech argued that “[n]either Thia nor Tanenbaum discloses a flow re-assembler that is part of the NIC.”
The Board recited some of the parties’ arguments and concluded that the asserted prior art teaches the reassembly limitations. Alacritech argued that the Board’s analysis did not adequately support its obviousness finding. The Federal Circuit reviewed the adequacy of the Board’s obviousness finding under the APA’s requirement that the Board’s decisions be supported by adequate reasoning and evidence. The Federal Circuit emphasized that the Board’s findings need not be perfect, but must be “reasonably discernable.” However, the Board failed to meet the requirement by explaining why it found those arguments persuasive. The Federal Circuit reasoned that the Board briefly recites two terse paragraphs from the parties’ arguments and, in doing so, “appears to misapprehend both the scope of the claims and the parties’ arguments.” Further, the Federal Circuit turned down Intel’s argument that it should prevail because the Board “soundly rejected” Alacritech’s arguments. But the Board’s rejection does not necessarily support the Board’s finding that the asserted prior art teaches or suggests reassembly in the network interface. The Board should meet the obligation to “articulate a satisfactory explanation for its action including a rational connection between the facts found and the choice made.” The Federal Circuit, therefore, vacated the portion of the Board’s decision.
Alacritech successfully kept the ball rolling due to the Board’s inadequate reasoning and evidence. However, Alacritech did not substantially weaken the obviousness determination, and its teaching away argument on Tanenbaum hit the wall. Tanenbaum suggests on protocols for gigabit networks that “[u]sually, the best approach is to make the protocols simple and have the main CPU do the work.” Such a statement did not disparage other approaches. As pointed out by the Federal Court, “Tanenbaum merely expresses a preference and does not teach away from offloading processing from the CPU to a separate processor.”
The Federal Circuit affirmed the Board’s finding of a motivation to combine Thia and Tanenbaum, explaining that “[a] reference that ‘merely expresses a general preference for an alternative invention but does not criticize, discredit, or otherwise discourage investigation into’ the claimed invention does not teach away.” (Meiresonne v. Google (Fed. Cir. March 7, 2017)) The proposition makes clear that a general preference cannot support teaching away argument. A proper teach away argument requires evidence that shows the prior art references would lead away from the claimed invention.
Takeaway
- The PTAB’s obviousness findings should meet the APA’s requirements to support its decisions with adequate reasoning and evidence.
- A proper teach away argument requires evidence that shows the prior art references would lead away from the claimed invention.


