Satisfying Written Description When Therapeutic Effectiveness is Claimed
| June 26, 2019
Nuvo Pharmaceuticals v. Dr. Reddy’s Laboratories
Summary
The CAFC reversed and dismissed a holding by the District Court that the claims of the ‘907 and the ‘285 patents had adequate written description regarding the efficacy of an uncoated PPI. The CAFC states that it not necessary to prove that a claimed pharmaceutical compound actually achieves a certain result. However, if the claim recites said result, then there must be sufficient support in the specification. Herein, the claims were held invalid because the therapeutic effectiveness of uncoated PPI, which was recited in the claims, was not supported by the specification.
Details
The use of a non-steroidal anti-inflammatory drug (hereinafter “NSAID”), such as aspirin, can cause gastrointestinal problems, and thus, some patients are prescribed an acid inhibitor, such as proton pump inhibitor (PPI), to be taken with said NSAID. However, even this combination therapy may be problematic. That is, if the PPI has not taken affect before the administration of the NSAID then gastrointestinal problems may still occur.
The U.S. Patent Nos. 6,926,907 (hereinafter “the ‘907 patent”) and 8,557,285 (hereinafter “the ’285 patent”) are directed towards a coordinated release drug formulation comprising an acid inhibitor/PPI and a NSAID. The coordinated release drug allows for an acid inhibitor to work before the release of the NSAID and thereby minimizes potential gastrointestinal problems. The ‘285 patent is a related patent of the ‘907 patent and both share a specification. Claim 1 of the ‘907 patent and claim 1 of the ‘285 patent are presented below:
Claim 1 of the ’907 patent:
1. A pharmaceutical composition in unit dosage form suitable for oral administration to a patient, comprising:
(a) an acid inhibitor present in an amount effective to raise the gastric pH of said patient to at least 3.5 upon the administration of one or more of said unit dosage forms;
(b) a non-steroidal anti-inflammatory drug (NSAID) in an amount effective to reduce or eliminate pain or inflammation in said patient upon administration of one or more of said unit dosage forms;
and wherein said unit dosage form provides for coordinated release such that:
i) said NSAID is surrounded by a coating that, upon ingestion of said unit dosage form by said patient, prevents the release of essentially any NSAID from said dosage form unless the pH of the surrounding medium is 3.5 or higher;
ii) at least a portion of said acid inhibitor is not surrounded by an enteric coating and, upon ingestion of said unit dosage form by said patient, is released regardless of whether the pH of the surrounding medium is below 3.5 or above 3.5.
(emphasis added)
Claim 1 of the ’285 patent:
1. A pharmaceutical composition in unit dosage form comprising therapeutically effective amounts of:
(a) esomeprazole, wherein at least a portion of said esomeprazole is not surrounded by an enteric coating; and
(b) naproxen surrounded by a coating that inhibits its release from said unit dosage form unless said dosage form is in a medium with a pH of 3.5 or higher;
wherein said unit dosage form provides for release of said esomeprazole such that upon introduction of said unit dosage form into a medium, at least a portion of said esomeprazole is released regardless of the pH of the medium.
(emphasis added)
Nuvo, who owns the ‘907 and ‘285 patents, make and sells Vimovo, which is the commercial embodiment of the patents. The patented drug achieves a coordinated release of the acid inhibitor and the NSAID in a single tablet. The core of the tablet is NSAID, which is coated so as to prevent its release before the pH has increased to a desired level, and an acid inhibitor, like PPI, on the outside of the coating, that actively works to increase the pH to said desired level. The PPI is uncoated. The specifications discloses methods for preparing and making the claimed drug formulations and provides examples of the structure and ingredients of the drug formulations but does not disclose any experimental data demonstrating the therapeutic effectiveness of any amount of uncoated PPI and coated NSAID in a single dosage form. Id. at 6. The specification discloses that coated PPIs avoid destruction by stomach acid but may not work quickly enough and the specification does not have any disclosure regarding the effectiveness of uncoated PPIs being able to raise pH. The inventor of the ‘907 and ‘285 patents recognized that an uncoated PPI is at greater risk of being destroyed by stomach acid, which would undermine the effectiveness of the PPI, but contemplated that uncoated PPI would allow for immediate release into a patient’s stomach and achieve an increase in pH level.
Dr. Reddy’s Laboratories, Inc., Mylan Pharmaceuticals, and Lupin Pharmaceuticals (hereinafter “the Generics”) submitted an Abbreviated New Drug Applications (ANDAs) to the U.S. Food and Drug Administration seeking approval to sell a generic version of Vimovo. Dr. Reddy’s submitted a second ANDA wherein the product would contain a small amount of uncoated NSAID on the outermost layer of the tablet, which is separate from the coated-core-NSAID.
Nuvo sued the Generics in the U.S. District Court for the District of New Jersey, in order to prevent the Generics from entering the market upon approval of the ANDAs, alleging all ANDAs products would infringe the ‘907 and ‘285 patents. The Generics stipulated to infringement, except for Dr. Reddy’s second ANDA product, and then countered that the ‘907 and ‘285 patents were invalid for obviousness, lack of enablement and inadequate written description.
The District Court granted Dr. Reddy’s motion for summary judgment of noninfringment of the ‘907 patent with regards to the second ANDA product. A bench trail was held regarding the validity of the ‘907 patent, the ‘285 patent, and whether the second ANDA product by Dr. Reddy infringed the ‘285 patent. It was concluded that the claims were not obvious over the prior art “because it was nonobvious to use a PPI to prevent NSAID-related gastric injury, and persons of ordinary skill in the art were discouraged by the prior art from using uncoated PPI and would not have reasonably expected it to work.” Id. at 8. It was also held that the claims of both patents were enabled and there was sufficient written description. The District Court held that the second ANDA by Dr. Reddy infringes the claims of the ‘285 patent.
At the District Court, the Generics argued that, “if they lose on their obviousness contention, then the claims lack written description support for the claimed effectiveness of uncoated PPI because ordinarily skilled artisans would not have expected it to work and the specification provides no experimental data or analytical reasoning showing the inventor possessed an effective uncoated PPI.” Id. at 9. Nuvo countered that “experimental data and an explanation of why an invention works are not required, the specification adequately describes using uncoated PPI, and its effectiveness is necessarily inherent in the described formulation.” Id. at 9. The District Court rejected Nuvo’s argument that effectiveness does not need to be described because effectiveness is inherent. The District Court acknowledged that the specification of the ‘907 and ‘285 patents did not describe efficacy of uncoated PPI. However, the District Court did conclude that there was sufficient written description because “the specification described the immediate release of uncoated PPI and the potential disadvantages of coated PPI, namely that enteric-coated PPI sometimes works too slowly to raise the intragastric pH. The district court did not explain why the mere disclosure of immediate release uncoated PPI, coupled with the known disadvantages of coated PPI, is relevant to the therapeutic effectiveness of uncoated PPI, which the patent itself recognized as problematic for efficacy due to its potential for destruction by stomach acid.” Id. at 10. The Generics appeal the written description ruling and Nuvo cross-appeals the District Court grant of summary judgment of noninfringement. Based solely on the written description issue regarding the claim language of “efficacy”, the CAFC reversed the appeal and dismissed the cross-appeal.
Before the CAFC, the Generics argued that the patents claim uncoated PPI that raises the gastric pH to at least 3.5, but that in view of the District Court’s holding, as part of the obviousness analysis, a skilled artisan would not have expected uncoated PPI to be effective to raise gastric pH, and that the specification of the patents fails to disclose the effectiveness of uncoated PPI. Id. at 12. Nuvo argued that “the claims do not require any particular degree of efficacy of the uncoated PPI itself, it is enough that the specification discloses making and using drug formulations containing effective amounts of PPI and NSAID, and experimental data and additional explanations demonstrating the invention works are unnecessary.” Id. at 12. The CAFC held that the District Court’s analysis does not support its conclusion of adequate written description and gave a review of the record to establish that the clear error standard has been met. “A written description finding is review for a clear error.” Id. at 11.
First, the CACF rejected Nuvo’s argument that the claims do not recite an efficacy requirement for uncoated PPI. As noted above, claim 1 of the ‘907 patent discloses “an acid inhibitor present in an amount effective to raise the gastric pH of said patient to at least 3.5” and claim 1 of the ‘285 patent discloses “therapeutically effective amounts of (a) esomeprazole….” The CAFC held that the claims of both patents require an amount of uncoated PPI effective to raise the gastric pH to at least 3.5. Id. at 14. Further, the CAFC noted that Nuvo’s argument, which is an attempt to “either recharacterizing the written description dispute or rewriting the claim language”, is being presented for the first time on appeal and is thus forfeited. The CAFC held that, before the District Court, the parties characterized that “claims require a therapeutically effective amount of uncoated PPI that would raise the gastric pH to at least 3.5”, that this understanding was “a fair reading of the claim language” and this understanding will not be altered in the appeal. Id. at 16.
Next, Nuvo argued that the expert testimony of Dr. Williams identified four portions of the specification that provided written description support. The Generics argued that the noted portions only disclose typical dosage amounts of uncoated PPI, the use of uncoated PPI in a drug formulation and did not discuss or explain efficacy of uncoated PPI. The CAFC agreed with the Generics. “We have expressly rejected the “argument that the written description requirement … is necessarily met as a matter of law because the claim language appears in ipsis verbis in the specification.” Enzo Biochem, Inc. v. GenProbe Inc., 323 F.3d 956, 968 (Fed. Cir. 2002).” Id. at 18. The CAFC noted that the case law does not requirement experimental data to establish effectiveness or an explanation of how or why a claimed composition will be effective. Id. at 18. Nevertheless, the CAFC held that the “record evidence demonstrates that a person of ordinary skill in the art would not have known or understood that uncoated PPI is effective.” Id. at 18. The CAFC held that the specification is fatally flawed since the “the specification provides nothing more than the mere claim that uncoated PPI might work, even though persons of ordinary skill in the art would not have thought it would work…. It does not demonstrate that the inventor possessed more than a mere wish or hope that uncoated PPI would work, and thus it does not demonstrate that he actually invented what he claimed: an amount of uncoated PPI that is effective to raise the gastric pH to at least 3.5” Id. at 19. The inventor’s own testimony confirms this holding. At trial, the inventor admitted “that he only had a ‘general concept of coordinated delivery with acid inhibition’ using uncoated PPI at the time he filed his first patent application.” Id. at 19.
Lastly, Nuvo argued that the written description requirement was satisfied due to the disclosure of how to make and use the claimed invention and accept that therapeutic effectiveness of uncoated PPI is a matter of inherency. Id. at 20 and 21. The Generics argued that Nuvo’s assertion did not satisfy written description but would only satisfy the enablement requirement, which is a separate and distinct requirement. Nuvo cited Alcon Research Ltd. v. Barr Laboratories, Inc., 745 F.3d 1180 (Fed. Cir. 2014) for support but the CAFC quickly dismissed this and noted that the factual circumstances of Alcon were “markedly different”. Id. at 22. In Alcon, the patent reference presented example formulations and disclosed data showing stability testing of the claimed invention. Further, the CAFC stated that only “under a narrow set circumstance” would inherency satisfy the written description requirement. Nuvo cited Allergan to support their argument that the claimed efficacy of uncoated PPI is necessarily inherent in the specification’s explicit disclosure of methods for making and using drug formulations containing uncoated PPI. The CAFC agreed with the Generics that the factual circumstances of Allergan are not applicable to the present case. In Allergan, the parties did not dispute the therapeutic efficacy of the claimed formulation and the specification in Allergan presented experimental results that established a trend in clinical effectiveness.
Based on the specific facts of certain cases, it is unnecessary to prove that a claimed pharmaceutical compound actually achieves a certain result. But when the inventor expressly claims that result, our case law provides that that result must be supported by adequate disclosure in the specification. In this case, the inventor chose to claim the therapeutic effectiveness of uncoated PPI, but he did not adequately describe the efficacy of uncoated PPI so as to demonstrate to ordinarily skilled artisans that he possessed and actually invented what he claimed. And the evidence demonstrates that a person of ordinary skill in the art reading the specification would not have otherwise recognized, based on the disclosure of a formulation containing uncoated PPI, that it would be efficacious because he or she would not have expected uncoated PPI to raise gastric pH. Under those facts, the patent claims are invalid for lack of adequate written description pursuant to § 112, ¶ 1.
(emphasis added). Id. at 24.
The CAFC holds that the ‘907 patent and the ‘285 patent invalid for lack of adequate written description with regards to the claimed effectiveness of uncoated PPI. The CAFC did not address the other issues on appeal and cross-appeal.
Takeaway
- Before
filing an application, one may consider identifying the written description support
in the specification for each individual feature of a claim
- Given the narrow set of circumstances, try not to rely upon inherency to satisfy written description
- If possible, include experimental data of drug formulations
A treatment method including an administering step based on discovery of a natural law is patentable eligible
| June 18, 2019
Endo pharmaceuticals, Et Al. v. Teva pharmaceuticals Et Al.
Summary
The Federal Circuit reversed the district court’s decision holding the claims of U.S. Patent No. 8,808,737 ineligible under 35 U.S.C. §101. The Federal Circuit held that the claims at issue are not directed to a natural law.
Details
Endo owns the ‘737 patent, entitled “method of treating pain utilizing controlled release oxymorphone pharmaceutical compositions and instruction on dosing for renal impairment.” The inventors of the ‘737 patent discovered that patients with moderately or severely impaired kidney function need less oxymorphone than usual to achieve a similar level of pain management. Accordingly, the treatment method of the ‘737 patent advantageously allows patients with renal impairment to inject less oxymorphone while still treating their pain.
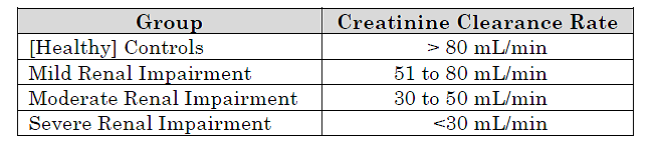
More specifically, the degree of renal impairment of the subjects can be indicated by their creatinine clearance rate. The subjects may be separated into four groups based on their creatinine clearance rates:
Furthermore, the inventors discover that there was a statistically significant correlation between plasma AUC (area under curve) for oxymorphone and a patient’s degree of renal impairment, as shown below:
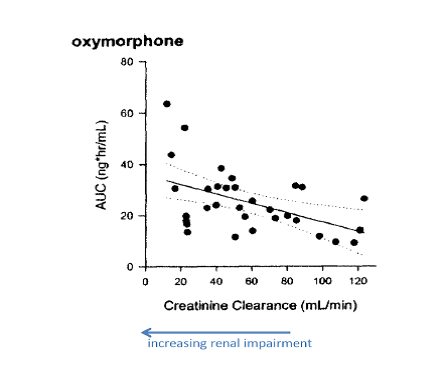
That is, there was relatively little change in oxymorphone AUC until the subjects had moderate-to-severe renal impairment (creatinine clearance rates below 50 mL/min). Subjects with severe renal impairment (creatinine clearance rates below 30 mL/min) had the highest AUC values.
Claim 1 of the ‘737 patent is as follows:
1. A method of treating pain in a renally impaired patient, comprising the steps of:
a. providing a solid oral controlled release dosage form, comprising:
a) about 5 mg to about 80 mg of oxymorphone or a pharmaceutically acceptable salt thereof as the sole active ingredient; and
b) a controlled release matrix;
b. measuring a creatinine clearance rate of the patient and determining it to be
a) less than about 30 ml/min;
b) about 30 mL/min to about 50 mL/min;
c) about 51 mL/min to about 80 mL/min, or
d) about 80 mL/min; and
c. orally administering to said patient, in dependence on which creatinine clearance rate is found, a lower dosage of the dosage form to provide pain relief;
wherein after said administration to said patient, the average AUC of oxymorphone over a 12-hour period is less than about 21 ng·hr/mL.
The magistrate judge held, and the district court agreed, that the claims at issue were not patent-eligible because the claims are directed to the natural law that the bioavailability of oxymorphone is increased in people with severe renal impairment, and the three steps a-c do not add “significant more” to qualify as a patentable method.
However, the federal circuit disagrees with the district court, holding that the claims at issue were directed to a patent-eligible application of a natural law. Specifically, The Federal Circuit points out that “it is not enough to merely identify a patent-ineligible concept underlying the claim; we must determine whether that patent-ineligible concept is what the claim is ‘directed to.’” Applying this law, the Federal Circuit concluded that claims of the ‘737 patent are directed to a patent-eligible method of using oxymorphone or a pharmaceutically acceptable salt thereof to treat pain in a renally impaired patient. The conclusion is supported by the claim language itself and confirmed by the specification. The claim language recites specific steps a-c in using oxymorphone to treat pain in a renally impaired patient. The specification predominantly describes the invention as a treatment method, and explains that the method avoids possible issues in dosing and allows for treatment with the lowest available dose for patients with renal impairment. That is, the inventors here recognized the relationship between oxymorphone and patients with renal impairment, but claimed an application of that relationship, which is a method of treatment including specific steps to adjust or lower the oxymorphone dose for patients with renal impairment.
Then, the Federal Circuit considered that the claims at issue are similar to those in Vanda Pharmaceuticals Inc. v. West-Ward Pharmaceuticals International Ltd, (Fed. Cir. 2018) and Rapid Litig. Mgmt. Ltd. V. CellzDirect (Fed. Cir. 2016) while distinguishing the claims at issue from those in Mayo collaborative Servs. V. Prometheus Labs., Inc. (SC 2012), and Ariosa Diagnostics, Inc. v. Sequenom, Inc., (Fed. Cir. 2015). For example, the claims at issue in Vanda related to a method of treating schizophrenia patients with a drug (iloperidone), where the administered dose is adjusted based on whether or not the patient is a “CYP2D6 poor metabolizer.” Like the claims in Vanda, the claims at issue here “are directed to a specific method of treatment for specific patients using a specific compound at specific doses to achieve a specific outcome.” In contrast, the representative claim in Mayo recited administering a thiopurine drug to patient as a first step in the method before determining the natural law relationship. The administering step is not performed based on the natural law relationship, and accordingly is not an application of the natural law.
Take away
- Adding an application step such as an administering step based on discovery of a natural law will render the claims patent eligible.
- There is a potential problem of divided infringement issue here in the subsequent enforcement effort. But direct infringement against physicians and induced infringement against pharmaceutical companies have been found based on similar patent claims. Eli Lilly & Co. v. Teva Parenteral Medicines, Inc., Appeal No. 2015-2067 (Fed. Cir. Jan. 12, 2017).
