File your patent application before attending a trade show to showcase your products
| May 26, 2023
Minerva Surgical, Inc. v. Hologic, Inc., Cytyc Surgical Products, LLC
Decided: February 15, 2023
Summary:
Minerva Surgical, Inc. sued Hologic, Inc. and Cytyc Surgical Products, LLC in the District of Delaware for infringement of U.S. Patent No. 9,186,208 (“the ’208 patent”). Hologic moved for summary judgment of invalidity, arguing that the ’208 patent claims were anticipated under the public use bar of pre-AIA 35 U.S.C. § 102(b). The district court granted summary judgment that the asserted claims are anticipated under the public use bar of pre-AIA 35 U.S.C. § 102(b) because the patented technology was “in public use,” and the technology was “ready for patenting.” The Federal Circuit held that the district court correctly determined that Minerva’s disclosure of their constituted the invention being “in public use,” and that the device was “ready for patenting.” Therefore, the Federal Circuit affirmed the district court’s grant of summary judgment.
Details:
Minerva Surgical, Inc. sued Hologic, Inc. and Cytyc Surgical Products, LLC in the District of Delaware for infringement of U.S. Patent No. 9,186,208 (“the ’208 patent”).
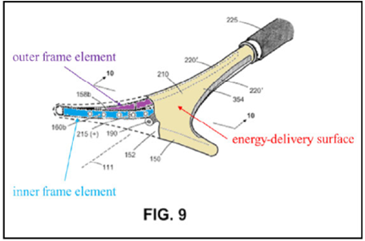
The ’208 patent is directed to surgical devices for a procedure called “endometrial ablation” for stopping or reducing abnormal uterine bleeding. This procedure includes inserting a device having an energy-delivery surface into a patient’s uterus, expanding the surface, energizing the surface to “ablate” or destroy the endometrial lining of the patient’s uterus, and removing the surface.
The application for the’208 patent was filed on November 2, 2021 and claims a priority date of November 7, 2011. Therefore, the critical date for the ’208 patent is November 7, 2010.
The ’208 patent
Independent claim 13 is a representative claim:
A system for endometrial ablation comprising:
an elongated shaft with a working end having an axis and comprising a compliant energy-delivery surface actuatable by an interior expandable-contractable frame;
the surface expandable to a selected planar triangular shape configured for deployment to engage the walls of a patient’s uterine cavity;
wherein the frame has flexible outer elements in lateral contact with the compliant surface and flexible inner elements not in said lateral contact, wherein the inner and outer elements have substantially dissimilar material properties.
The appeal focused on the claim term, “the inner and outer elements have substantially dissimilar material properties” (“SDMP” term”).
District Court
After discovery, Hologic moved for summary judgment of invalidity, arguing that the ’208 patent claims were anticipated under the public use bar of pre-AIA 35 U.S.C. § 102(b).
The district court granted summary judgment that the asserted claims are anticipated under the public use bar of pre-AIA 35 U.S.C. § 102(b) because of the following reasons:
First, the patented technology was “in public use” because Minerva disclosed fifteen devices (“Aurora”) at an event, where Minerva showcased them at a booth, in meeting with interested parties, and in a technical presentation. Also, Minerva did not disclose them under any confidentiality obligations.
Second, the technology was “ready for patenting” because Minerva created working prototypes and enabling technical documents.
Federal Circuit
The Federal Circuit reviewed a district court’s grant of summary judgment under the law of the regional circuit (Third Circuit).
The Federal Circuit held that “the public use bar is triggered ‘where, before the critical date, the invention is [(1)] in public use and [(2)] ready for patenting.’”
The “in public use” element is satisfied if the invention “was accessible to the public or was commercially exploited” by the invention.
“Ready for patenting” requirement can be shown in two ways – “by proof of reduction to practice before the critical date” and “by proof that prior to the critical date the inventor had prepared drawings or other descriptions of the invention that were sufficiently specific to enable a person skilled in the art to practice the invention.”
The Federal Circuit held that disclosing the Aurora device at the event (American Association of Gynecologic Laparoscopists (“AAGL 2009”)) constituted the invention being “in public use” because this event included attendees who were critical to Minerva’s business, and Minerva’s disclosure of their devices included showcasing them at a booth, in meeting with interested parties, and in a technical presentation.
The Federal Circuit noted that AAGL 2009 was the “Super Bowl” of the industry and was open to the public, and that Minerva had incentives to showcase their products to the attendees. Also, Minerva sponsored a presentation by one of their board members to highlight their products and pitched their products to industry members, who were able to see how they operate.
The Federal Circuit also noted that there were no “confidentiality obligations imposed upon” those who observed Minerva’s devices, and that the attendees were not required to sign non-disclosure agreements.
The Federal Circuit also held that Minerva’s Aurora devices at the event disclosed the SDMP term because Minerva’s documentation about this device from before and shortly after the event disclosed this device having the SDMP terms or praises benefits derived from this device having the SDMP technology.
The Federal Circuit held that the record clearly showed that Minerva reduced the invention to practice by creating working prototypes that embodied the claim and worked for the intended purpose.
The Federal Circuit noted that there was documentation “sufficiently specific to enable a person skilled in the art to practice the invention” of the disputed SDMP term. Here, the documentation included the drawings and detailed descriptions in the lab notebook pages disclosing a device with the SDMP term.
Therefore, the Federal Circuit held that the district court correctly determined that Minerva’s disclosure of the Aurora device constituted the invention being “in public use” and that the device was “ready for patenting.”
Accordingly, the Federal Circuit affirmed the district court’s grant of summary judgment because there are no genuine factual disputes, and Hologic is entitled to judgment as a matter of law that the ’208 patent is anticipated under the public use bar of § 102(b).
Takeaway:
- File your patent application before attending a trade show to showcase your products.
- Have the attendees of the trade show sign non-disclosure agreements, if necessary.
Tags: 35 U.S.C. § 102(b) > confidentiality > critical date > enablement > pre-AIA > prototype > public use > reduction to practice > summary judgment
Under pre-AIA law, conception of a DNA segment is established by possession and appreciation, not completely accurate sequence knowledge
| November 20, 2013
Sanofi-Aventis v Pfizer, Inc.
November 5, 2013
Before Newman (Circuit Judge), Lourie (Circuit Judge) and Davis (District Judge). Opinion by Newman.
Summary:
A patent is awarded to the first party to conceive and reduce to practice the invention. When the invention is directed towards a DNA segment, conception requires possession and appreciation of the DNA segment that is claimed. A completely correct sequence of the DNA segment is not the standard for establishing conception and reduction to practice.
Tags: conception > nucleotide > reduction to practice > sequence
Non-enabling disclosure by first inventor of low defect single crystal was sufficient to defeat under 102(g) patent claim of subsequent inventor
| December 12, 2012
The Fox Group, Inc. v. Cree, Inc.
November 28, 2012
Panel: Newman, O’Malley, and Wallach. Opinion by Wallach. Dissent by O’Malley.
Summary:
(1) Summary judgment in favor of defendant was affirmed with respect to asserted claims because (a) defendant was first to reduce to practice the claimed SiC single crystal, and (2) plaintiff did not produce sufficient evidence raising genuine issue of material fact to show that defendant suppressed or concealed the invention.
(2) Holding that unasserted claims were invalid was vacated.
Read More/続きを読む