Patent Term Extension under 35 U.S.C. §156 is Limited to the FDA-Approved Active Ingredient and Salts or Esters thereof—but not Metabolites or Compounds Sharing an Active Moiety
| May 13, 2020
Biogen International GmbH v. Banner Life Sciences
April 21, 2020
Lourie, Moore, and Chen. Opinion by Lourie
Summary
In a case focusing on the meaning of a “product” under 35 U.S.C. §156, the CAFC concluded that the scope of patent term extension is limited to the FDA-approved active ingredient and salts or ester thereof, and does not more broadly include metabolites or other compounds sharing an active moiety with the approved product. Thus, the extension of Biogen’s ‘001 patent does not include some other products within the scope of the claims, such as the metabolite MMF.
Details
Background and District Court holding
Biogen is the owner of U.S. Patent No. 7,619,001, which claims a method of treating multiple sclerosis. Claim 1 is as follows:
A method of treating multiple sclerosis comprising administering, to a patient in need of treatment for multiple sclerosis, an amount of a pharmaceutical preparation effective for treating multiple sclerosis, the pharmaceutical preparation comprising
at least one excipient or at least one carrier or at least one combination thereof; and
dimethyl fumarate, methyl hydrogen fumarate, or a combination thereof.
Biogen also holds the New Drug Application (NDA) for dimethyl fumarate (DMF). This was approved by the FDA in 2013 and is marketed as Tecfidera® “for the treatment of patients with relapsing forms of multiple sclerosis” at a dose of 480 mg.
DMF is a double ester which, upon administration to the patient, metabolizes into monomethyl fumarate (MMF) (another name for the claimed “methyl hydrogen fumarate”). Both DMF and MMF are illustrated below.
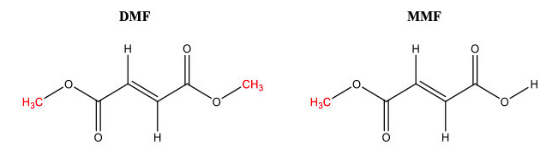
The ‘001 patent is was originally set to expire on April 1, 2018, but was awarded 818 days of patent term extension to June 20, 2020 under 35 U.S.C. 156 (not to be confused with patent term adjustment under 35 U.S.C. 154). This compensated Biogen for the FDA review period of Tecfidera. The question presented to the CAFC is whether MMF is covered by the 818-day PTE extension along with DMF.
In 2018, Banner filed a “paper NDA” under 21 U.S.C. 355(b)(2) to market a drug having MMF as the active ingredient. Banner could rely on Biogen’s clinical data to show safety and efficacy, and only had to demonstrate bioequivalence. Biogen then sued Banner for infringement.
In reply, Banner argued that the PTE under §156 was limited to uses including the approved product: DMF and its salts or esters, and did not include MMF. The relevant portions of §156 are presented below:
(a) The term of a patent which claims a product, a method of using a product, or a method of manufacturing a product shall be extended in accordance with this section from the original expiration date of the patent, which shall include any patent term adjustment granted under section 154(b),…
(b) Except as provided in subsection (d)(5)(F), the rights derived from any patent the term of which is extended under this section shall during the period during which the term of the patent is extended—….
(2) in the case of a patent which claims a method of using a product, be limited to any use claimed by the patent and approved for the product—
(A) before the expiration of the term of the patent—
(i) under any provision of law under which an applicable regulatory review occurred, and
(ii) under the provision of law under which any regulatory review described in paragraph (1), (4), or (5) of subsection (g) occurred, and
(B) on or after the expiration of the regulatory review period upon which the extension of the patent was based; and….
(f) For purposes of this section:
(1) The term “product” means:
(A) A drug product…..
(2) The term “drug product” means the active ingredient of—
(A) a new drug, antibiotic drug, or human biological product…including any salt or ester of the active ingredient, as a single entity or in combination with another active ingredient.
Meanwhile, Biogen argued that §156(b)(2) does not limit the PTE to uses of the approved product, but rather to all products within the scope of the claim. Additionally, Biogen argued that “product” of §156 should be more broadly interpreted as any compound that shares the “active moiety” with the approved product. The district court sided with Banner.
CAFC
First, the CAFC noted that the purpose of §156 PTE is to compensate a patent owner for regulatory delays, and that this is limited to one patent per approved product. The court also noted that §156 defines the scope of the product as “the active ingredient of…a new drug…including any salt or ester of the active ingredient.”
Biogen cited Pfizer Inc. v. Dr. Reddy’s Labs, Ltd. 359 F.3d 1361 (Fed. Cir. 2004) to support the position that that “product” under §156 should be interpreted more broadly, especially where “a later applicant’s patentably indistinct drug product…relies on the patentee’s clinical data.” The CAFC did not accept this argument. In Pfizer, the question was whether an extension for amlodipine included amlodipine maleate. However, the CAFC concluded that amlodipine maleate in Pfizer was included under the extension because amlodipine maleate is a salt of the active ingredient (amlodipine), and not because these two compounds share an “active moiety.” On the other hand, in this case, MMF is not a salt or ester of DMF. Rather, MMF is a de-esterified form of DMF. The CAFC’s comments regarding clinical data were provided in Pfizer merely to clarify the purpose of §156(f) and provide context.
The parties also cited to Glaxo Ops. UK Ltd. v. Quigg, 894 F.2d 392 (Fed. Cir. 1990). However, the CAFC concluded that this case was not pertinent to the present issue. Glaxo involved an approved product having cerufoxime as its active ingredient and a second approved product having cerufoxime axetil as its active ingredient, cerufoxime axetil being an ester of cerufoxime. In Glaxo, the question was not whether cerufoxime axetil should be included in an extension for cerufoxime, but rather whether cerufoxime axetil was eligible for its own separate PTE. Since this is not the issue in the present case, the CAFC side-stepped Glaxo.
Additionally, Biogen cited to PhotoCure ASA v. Kappos, 603 F.3d 1372 (Fed. Cir. 2004). In PhotoCure, the CAFC indicated that a new ester could be separately patentable. However, the CAFC indicated that PhotoCure presented a similar situation as Glaxo: a separate extension for a new ester.
Thus, the CAFC held that the definition of “product” according to §156 is the “active ingredient ….including any salt or ester of the active ingredient.” This is not necessarily the same as “active moiety.” As such, “product” only means the active ingredient designated on the FDA approved label, and changes resulting in a salt or ester. However, “product” does not include metabolites of the active ingredient, or de-esterified forms thereof. Here, MMF is a de-esterified metabolite of DMF, rather than a salt of DMF.
Next, Biogen argued that, unlike §156(b)(1), §156(b)(2) does not limit the extension to approved uses of the approved product, but only to approved uses of any approved product. However, the CAFC dismissed this by holding that the approved product in this case is DMF, not MMF, and that it would not make sense for an extension to apply to a different product for which the NDA holder was never subjected to review.
Finally, Biogen argued that the Banner infringes under the doctrine of the equivalents. However, the CAFC dismissed this argument on that basis that a product or process cannot logically infringe an extended claim under equivalents if it is statutorily not included in the §156 extension.
Take Away
Unlike patent term adjustment under §154, which covers the full scope of the patent, patent term extension under §156 is limited in scope to a product having the active ingredient granted regulatory approval by the FDA. The “product” will be narrowly interpreted as the approved active ingredient, as well as salts and esters thereof. As such, patent term extension under §156 is not so broad to cover metabolites and other compounds which share an active moiety with the approved drug.
Interestingly, this case was decided near in time to an important IPR involving the same drug. In February, the PTAB sided with Biogen in a challenge to U.S. Patent No. 8,399,514 by Mylan Pharmaceuticals. The ‘514 patent was very similar to the claims of the ‘001 patent, but recited oral administration and a specific dosage. The PTAB indicated that the dosage was not obvious from the prior art and thus confirmed the validity of the valuable ‘514 patent. The ‘514 patent is the last unexpired patent in the family covering Tecfidera, and is set to expire in 2028.
Additionally, upon the CAFC’s decision, the FDA granted final approval on April 30 to Banner’s MMF-based drug, which will be marketed as Bafiertam ®.


