Beware of Relying on a Single Example, Since It May Limit Claim Scope
| October 17, 2013
Sunovion Pharmaceuticals, Inc. v. Teva Pharmaceuticals, et al.
September 26, 2013
Panel: Lourie, Schall and Reyna. Opinion by Lourie
Summary
In this case arising from an ANDA, the claims recited the vague term of “essentially free of”, which was undefined by the specification. Although probably never intended to limit the scope of the claims, the CAFC held that the content of the sole substantive example in the specification and a declaration citing this—which was heavily relied upon during prosecution—defined the scope of this ambiguous term. However, the patent owner managed to win the litigation nonetheless, due to the CAFC recognizing the ineffectiveness of an unconventional “certification” of non-infringement to the district court which contradicted the defendant’s FDA filing.
Details
Many pharmaceutical compounds are available as enantiomers, which are non-superimposable mirror images of each other. These are called dextrorotatory and levorotatory isomers. For those unfamiliar with the concept, it is simplest to think of these as “right-handed” and “left-handed” versions of the same molecule. Often, these will have different properties, and it is often not possible to entirely separate one from another in a mixture. In this case, the levorotatory isomer can be thought of as an undesirable impurity.
Sunovion developed a drug which was eventually marketed as “Lunesta”, a sleep aid, and is the owner of U.S. Patent No. 6,444,673. The representative claim 1 of the ‘673 patent recites:
6-(5-chloro-2-pyridyl)-5-[(4-methyl-1-piperazinyl)carbonyloxy]-7-oxo-6,7-dihydro-5H-pyrrolo[3,4-b]pyrazine, or a pharmaceutically acceptable salt thereof, in the form of its dextrorotatory isomer and essentially free of its levorotatory isomer.
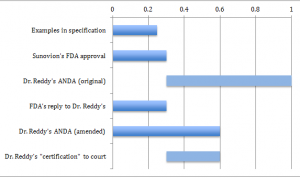
Nowhere in the specification or claims was there a definition of what was meant by “essentially free of”. The specification only indirectly notes that the invention is more than 50% of the dextrorotatory isomer. However, during prosecution, Sunovion stressed in a declaration and corresponding arguments that Example 1 of the specification contained less than 0.25% of the levorotatory isomer. Later, the FDA approved Lunesta based on the requirement that the marketed product can include no more than 0.3% of the levorotatory isomer.
Seeking to manufacture generic Lunesta, Dr. Reddy’s later filed an ANDA, including a Paragraph IV certification that there was no infringement of the ‘673 patent. Sunovion then initiated suit.
Dr. Reddy’s initial ANDA filing to the FDA sought approval for a formulation with not less than 0.3% but not more than 1.0% of the levorotatory isomer. But the FDA denied this, and told Dr. Reddy’s to revise their application such that the levorotatory isomer is not more than 0.3% of the formulation. Dr. Reddy’s later amended their ANDA application by requesting approval for a formulation with not more than 0.6% of the levorotatory isomer. This amended ANDA application has not yet been approved.
In a Markman hearing, claim construction was required of the term “essentially free of”. Based on the declaration during prosecution, the district court held that “essentially free of” means up to 0.25% of the levorotatory isomer. Additionally at the district court, Dr. Reddy’s moved for summary judgment of non-infringement based on a “certification” that they would not market the product containing less than 0.3% of the levorotatory isomer, and thus would not infringe the scope of the claims in view of the claim construction. The district court bought into this “certification”, and granted summary judgment of non-infringement. The chart below summarizes the ranges in question.
On appeal, the CAFC first addressed the claim construction. For reasons that are not particularly clear, Sunovion argued for an interpretation of “essentially free of” to mean up to 10% of the levorotatory isomer. However, the CAFC noted the lack of a plain meaning of the term, and the lack of a specific definition. The court then turned to the intrinsic record, which included repeated references a formulation with less than 0.25% of the levorotatory isomer. This was not only in a declaration explaining an Example during prosecution to overcome an obviousness rejection, but also in the course of an interference. Based on this, the CAFC saw no reason to change the district court’s claim construction.
However, the CAFC reversed the district court’s holding of non-infringement based on Dr. Reddy’s “certification”. In Hatch-Waxman litigation, the filing of the ANDA is the infringing act. Therefore, the potentially infringing product is defined by the content of the ANDA filing at the FDA. Any comment or promise to another entity does not change the fact that Dr. Reddy’s applied for regulatory approval of a generic during with not more than 0.6% of the levorotatory isomer (despite the FDA asking for not more than 0.3%). The scope of the ANDA is therefore partially within the scope of Sunovion’s claim scope of not more than 0.25% of the levorotatory isomer. As such, the CAFC reversed the holding of non-infringement. In short, the CAFC stated that a promise of “But I won’t do it” is insufficient to avoid infringement.
Takeaway
For those involved in prosecution, the main takeaway here is that when there is no clear definition of vague terms like “essentially free of”, it is very possible that these terms may be limited to data of the examples. This is especially true if there is only a single example, and if the example is relied upon in prosecution. It seems likely that the same result would occur even if the specification and prosecution history was peppered with guarded language like “in a preferred example”, “for example”, and the like. If possible, including remarks explaining more than a single possibility for a range can be helpful, even if the additional possibilities are merely prophetic.
Sunovion might have been able to get a broader claim construction of if they included a claim reciting “A mixture consisting essentially of the dextrorotatory isomer of [the drug]”. This is because “consisting essentially of” has a legal definition as including the elements that follow and materials that do not materially affect the basic and novel characteristics of the compound. Particularly based on the FDA’s requirements, it is conceivable that such a claim would have a slightly broader scope, perhaps including not more than 0.3% of the levorotatory isomer, rather than the slightly narrower scope of not more than 0.25% of the levorotatory isomer.
As to the litigation aspect of this case, when the litigation outcome hinges on the content of filing at a regulatory agency, any kind of contradictory “certification” or promise to a court is meaningless. While the district court was fooled by this, the CAFC saw right through it.